DNA synthesis in vitro : proceedings of the Second Annual Harry Steenbock Symposium held in Madison, Wisconsin on July 10-12, 1972 / edited by R.D. Wells and R.B. Inman.
- Harry Steenbock Symposium
- Date:
- ©1973, [1974]
Licence: Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)
Credit: DNA synthesis in vitro : proceedings of the Second Annual Harry Steenbock Symposium held in Madison, Wisconsin on July 10-12, 1972 / edited by R.D. Wells and R.B. Inman. Source: Wellcome Collection.
91/512 (page LXXXVII)
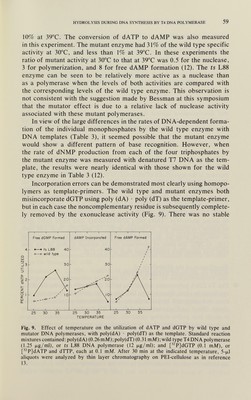
![HYDROLYSIS DURING DNA SYNTHESIS BY T4 DNA TOLYMERASE 59 10% at 39°C. The conversion of dATP to dAMP was also measured in this experiment. The mutant enzyme had 31% of the wild type specific activity at 30°C, and less than 1% at 39°C. In these experiments the ratio of mutant activity at 30°C to that at 39°C was 0.5 for the nuclease, 3 for polymerization, and 8 for free dAMP formation (12). The ts L88 enzyme can be seen to be relatively more active as a nuclease than as a polymerase when the levels of both activities are compared with the corresponding levels of the wild type enzyme. This observation is not consistent with the suggestion made by Bessman at this symposium that the mutator effect is due to a relative lack of nuclease activity associated with these mutant polymerases. In view of the large differences in the rates of DNA-dependent forma¬ tion of the individual monophosphates by the wild type enzyme with DNA templates (Table 3), it seemed possible that the mutant enzyme would show a different pattern of base recognition. However, when the rate of dNMP production from each of the four triphosphates by the mutant enzyme was measured with denatured T7 DNA as the tem¬ plate, the results were nearly identical with those shown for the wild type enzyme in Table 3 (12). Incorporation errors can be demonstrated most clearly using homopo- lymers as template-primers. The wild type and mutant enzymes both misincorporate dGTP using poly (dA) • poly (dT) as the template-primer, but in each case the noncomplementary residue is subsequently complete¬ ly removed by the exonuclease activity (Fig. 9). There was no stable 25 30 35 25 30 35 25 30 35 TEMPERATURE Fig. 9. Effect of temperature on the utilization of dATP and dGTP by wild type and mutator DNA polymerases, with poly(dA) • poly(dT) as the template. Standard reaction mixtures contained: poly(dA) (0.26mM);poly(dT) (0.31 rnM);wild type T4DNA polymerase (1.25 jjLg/ml), or ts L88 DNA polymerase (12 jjig/ml); and [PjdGTP (0.1 mM), or [P]dATP and dTTP, each at 0.1 mM. After 30 min at the indicated temperature, 5-(xl aliquots were analyzed by thin layer chromatography on PEI-cellulose as in reference 13.](https://iiif.wellcomecollection.org/image/b18037288_0092.JP2/full/800%2C/0/default.jpg)