Studies on the proteids of rye and barley and on the chemical nature of diastase.
- Thomas Burr Osborne
- Date:
- [1893]
Licence: Public Domain Mark
Credit: Studies on the proteids of rye and barley and on the chemical nature of diastase. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
7/64 (page 151)
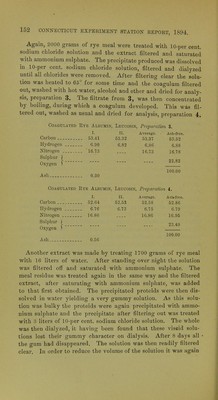
![Another extract was examined in a slightly difierent way. 1000 grams of rye meal were extracted with 11 liters of 10 per cent, sodium chloride solution and, in order to get rid of the laige amount of gum taken up, the solution, after filtering, was dialyzed and then saturated with ammonium sulphate. The precipitate thus produced was dissolv'ed, as far as possible in 10-per cent, sodium chloride brine, filtered clear and dialyzed until chlorides were removed. The solution after filtering clear was then heated to 65° and the albumin that separated was filtered out, washed thoroughly with hot water, with alcohol and with ether and dried over sulphuric acid. This preparation, 2j weighed 1.21 grams and had the following composition : Coagulated Rye Albumin, Lbucosin, Preparation 2. Carbon 53.04 Hydrogen 6.70 Nitrogen 16.57 Sulphur ) Oxygen ) Ash 0.50 The solution containing the proteoses, filtered from preparation 2, was then treated with 20 per cent, of its weight of dry sodium chloride and a little two-tenths per cent, hydrochloric acid was added which gave a considerable precipitate. This was filtered out, dissolved in distilled water and the solution dialyzed till free from chlorides. This solution then gave a precipitate with nitric acid, which dissolved on warming and precipitated again on cool- ing. The solution concentrated to a syrup on a water bath was precipitated by pouring into absolute alcohol. The precipitate when dried over sulphuric acid weighed 0,41 gram or one-third as much as the albumin. The filtrate, from the precipitation of this substance [with 20 per cent, of sodium chloride and acid], was satu- rated with ammonium sulphate and the precipitate thus produced filtered out and dissolved in distilled water. With copper sul- phate and potash this substance gave a clear pink color. Its solu- tion gave no precipitate on adding nitric acid until it had been saturated with sodium chloride, when a slight precipitate fell. It • yielded no precipitate with copper sulphate. These reactions indi- cate that besides albumin the aqueous extract contains small quan- tities of proto- and deutero-proteose. AsU-free, 53.29 6.74 16.65 23.32 100.00](https://iiif.wellcomecollection.org/image/b22469771_0009.jp2/full/800%2C/0/default.jpg)