Volume 1
A text-book of human physiology : including histology and microscopical anatomy with special reference to the requirements of practical medicine / by L. Landois ; translated from the seventh German edition with additions by William Stirling.
- Landois, L. (Leonard), 1837-1902. Lehrbuch der Physiologie des Menschen. English
- Date:
- 1891
Licence: Public Domain Mark
Credit: A text-book of human physiology : including histology and microscopical anatomy with special reference to the requirements of practical medicine / by L. Landois ; translated from the seventh German edition with additions by William Stirling. Source: Wellcome Collection.
65/602 (page 25)
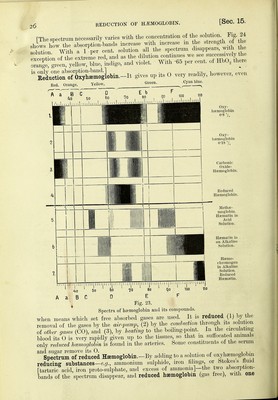
![dark to the obser ver. Oil account of this absorption, such a spectrum is called an ctbsovption spedrtmi. Flame Spectra.—If mineral substances be burned on a platinum-wire in a non-luminous flame or Bunsen's burner in front of the slit, the elements present in the mineral or ash give a special coloured band or bands, which have a definite position. Sodium gives a yellow, potassium a red and violet line. These substances are found on burning the ashes of almost all organs. If sunlight be allowed to fall upon the slit, the spectrum shows a large number of lines (Fraunhofer's lines) which occupy definite positions in the coloured spectrum. These lines are indicated by the letters A, B, C, D, &c.,. a, h, c, &c. (fig. 23). Fig. 22. Scheme of a spectroscope for observing the spectrum of blood. A, tube ; S, slit; m, m, layer of blood with flame in front of it; P, prism ; M, scale ; B, eye of observer looking through a telescope ; r', v', spectrum. 15. COMPOUNDS OF HB WITH 0; OXYHEMOGLOBIN AND METHiE- MOGLOBIN.—1. Oxyhsemoglobin (HbOg) behaves as a weak acid, and occurs to the extent of 86-78 to 94-30 per cent, in dry human red corpuscles {JUdell). It is formed very readily whenever Hb comes into contact with 0 or atmospheric air. According to Bohr, 1 gramme Hb unites with 1 -56 cubic centimetre of 0 at 0° and 760 mm. Hg pressure, the union being stronger in weak than in concentrated solutions. Oxyhaemoglobin is a very loose chemical compound, and is slightly less soluble than Hb; its spectrum shows in the yellow and the green two dark al)sorption-bands, whose length and breadth in a 0-18 per cent, solution are given in fig. 23 (2). It occurs in the blood-corpuscles circulating in arteries and capillaries, as can be shown by the spectroscopic examination of the ear of a rabbit, of the prepuce, and the web of the fingers (Vierordt). [Spectrum of Oxyhaemoglobin.—In the spectrum of a dilute solution of hsemo- globin crystals or arterial blood, part of the red and violet rays are absorbed, but two well-marked absorption-bands exist between D and E. The line nearest p, i.e., next the red end of the spectrum, sometimes designated by the letter (a) is narrow, sharply defined, and black at its centre, and its position corresponds to the wave-length 579. The other absorption-band near E, conveniently designated (^)j is broader, not so dark, and its edges are less sharply defined. Its centre corresponds to the wave-length 553-8. In very dilute solutions the a band is the only one visible. In a strong solution, as shown in fig. 23, the two bands fuse, but are again made visible as two on dilution of the blood.]](https://iiif.wellcomecollection.org/image/b20417688_001_0065.jp2/full/800%2C/0/default.jpg)