A manual of chemistry, inorganic and organic : inorganic and organic, with an introduction to the study of chemistry / by Arthur P. Luff.
- Arthur P. Luff
- Date:
- 1892
Licence: Public Domain Mark
Credit: A manual of chemistry, inorganic and organic : inorganic and organic, with an introduction to the study of chemistry / by Arthur P. Luff. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
54/558 (page 34)
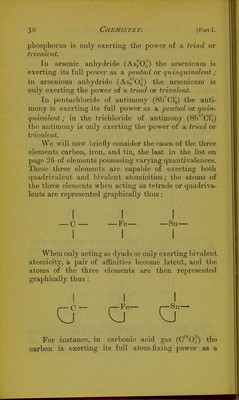
![0 1! N—O— 11 O Graphic representation of the radical of nitrates. (c) In the radical of nitrites (N'02) the nitrogen is only exerting trivalent atomicity (a pair of its affinities being latent), and is united with two bivalent oxygen atoms ; the three free affinities of the nitrogen atom will therefore saturate three out of the four affinities of the two oxygen atoms, leaving one affinity unsaturated, and therefore constituting the radical a univalent one. O II N—0— u Graphic representation of the radical of nitrites. {cl) In the radical of cyanides (C'^N^) an atom of the quadrivalent element carbon is united with an atom of the quinquivalent element nitrogen ; the four affinities of the carbon atom will therefore combine Avith four out of the five affinities of the nitrogen atom, leaving one affinity unsaturated, and so con- stituting the radical a univalent one. c=]sr— Graphic representation of the radical of cyanides. 2. As examples of bivalent acidulous radicals we will take the radicals of](https://iiif.wellcomecollection.org/image/b22651597_0054.jp2/full/800%2C/0/default.jpg)