First outlines of a dictionary of solubilities of chemical substances / by Frank H. Storer.
- Francis Humphreys Storer
- Date:
- 1864
Licence: Public Domain Mark
Credit: First outlines of a dictionary of solubilities of chemical substances / by Frank H. Storer. Source: Wellcome Collection.
Provider: This material has been provided by the National Library of Medicine (U.S.), through the Medical Heritage Library. The original may be consulted at the National Library of Medicine (U.S.)
33/742
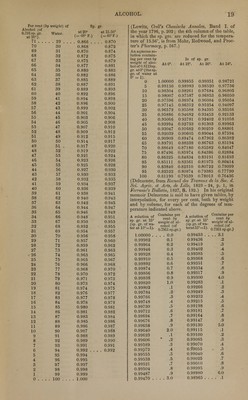
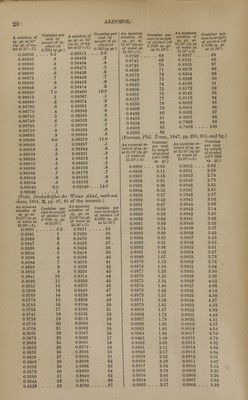
![iodides, bromides, and chlorides, and with certain oxygen salts. There are, however, certain excep- tions : thus, corrosive sublimate (HgCI) dissolves more abundantly in alcohol, especially in absolute alcohol, than in water, and, according to Kirwan, nitrate of magnesia dissolves more freely in alco- hol of 0.817 sp. gr. than in alcohol of 0.900 sp. gr. Compounds sparingly soluble in water are, for the most part, quite insoluble in alcohol; so likewise are efflorescent compounds. But all deliquescent compounds, excepting carbonate of potash, phos- phate of potash, and a few others, are soluble in alcohol. (Gmelin's Handbook, 8. 257.) Alcohol dissolves only those metallic oxides which possess either an alkaline or an acid char- acter. Of the metallic sulphides it dissolves only those of potassium and sodium ; of the iodides and bromides it dissolves a somewhat greater, and of the chlorides a still greater number. The car- bonates, borates, phosphites, phosphates, hyposul- phites, sulphites, hyposulphates, sulphates, iodates, and periodates, it dissolves either not at all or in very small quantity. The only sulphates which dissolve readily in alcohol are those of ferric and platinic oxide. On the other hand, alcohol dis- solves many hypophosphites, a still greater num- ber of bromates, chlorates, and perchlorates, and very many nitrates. (Gmelin, Ibid., p. 265.) With regard to the comportment of alcohol with organic compounds, the following general ob- servations may be made: — Alcohol dissolves all Hydrocarbons, and there- fore the primary Nuclei, and any compounds which those nuclei may form with hydrogen. — Among the compounds which likewise contain oxygen, alcohol dissolves especially those in which the number of atoms of all the elements together is comparatively small, and the oxygen is in com- paratively small proportion ; hence it is more in- clined to dissolve aldides (in which class may be included many volatile oils, camphors, and resins), and acids of small atomic weight, than acids of greater atomic weight, or richer in oxygen. Acids which are but slightly soluble or quite insoluble in alcohol likewise yield salts of similar character. Acids containing but little oxygen, and their salts, often dissolve in alcohol more readily than in wa- ter. Compounds in which hydrogen is replaced by iodine, bromine, or chlorine, do not appear to have their solubility in alcohol diminished by the substitution ; even the chlorides of carbon are all soluble in alcohol. All compounds of carbon, hydrogen, and nitrogen, e. g. the non-oxygenated alkaloids, are soluble in alcohol; but with regard to compounds of this nature containing oxygen, the observations above made concerning the influ- ence of oxygen likewise hold good. (Gmelin, loc. cit., p. 273.) Alcohol of 0.835 sp. gr. = 85%, called Drug- gists' Alcohol, is an excellent solvent for resins, camphor, benzoic acid, tannic acid, the balsams, grape-sugar, the vegetable alkalies, and castor-oil; also for iodine, carbonate of ammonia, chloride of ammonium, caustic potash or soda, nearly all deliquescent and a few other inorganic salts. It mixes freely in all proportions with water, ether, acetic acid, and most of the essential oils. Diluted Alcohol, of 0.935 sp. gr., consists of equal vols, of druggists' alcohol and water. It dissolves gums, vegetable albumen, and many col- oring matters; also, to a certain extent, resinous matters, essential oils, and vegetable alkalies; also sugar and tannic acid. (Parrish's Pharmacy, pp. 130, 131.) Dilute alcohol (a mixture of equal vols, alcohol 3 of 0.835 sp. gr. and of water) is a better solvent of resinous matters, and the extractive principles of plants, than the same quantity of these two liquids employed separately. (J. Personne, Amer. J. Pharm., 18. pp. 21, 103 ; cited by Parrish, Pharm., loc. cit.) An aqueous An aqueous solution con- solution con- taining per taining per cent,by vol., Is of cent,by vol.. Is of of alcohol of sp. gr. of alcohol of sp.gr. 0.7947 sp.gr. at 15°. 0.7947 sp.gr. at 15°. atl5°(thesp. atl5°(thesp. gr. of water gr. of water at 15° = 1). at 15° = 1). 100 .. . 0.7947 60 ... . 0.9141 95 0.8168 55 0.9248 90 0.8346 50 0.9348 85 0.8502 45 0.9440 80 0.8645 40 0.9523 75 0.8779 35 0.9595 70 0.8907 30 0.9656 65 . . . 2.9027 0 . . . . 1.0000 (Gay-Lussac, in Berzelius's Lehrb.) For the elaborate tables which Gay-Lussac has con- structed from these data we must refer the read- er to his treatise entitled Instruction pour I'usage de I'Alcoometre centesimal et des Tables qui Vac- compagnent. Paris, 1824; in Handmorterbuch der Chemie, 1% 235 et seq. See also Ure's Diet, of Arts. These tables which refer to the cen- tesimal alcohometer of their author [compare Maroseau's table below] indicate the percentage by volume of alcohol, reduced to 15°, for every degree of temperature from 0° to 30° C. A toler- ably close approximation to the figures of Gay- Lussac's table may be obtained by the formula of Francoeur (Ilandwiirterbuch, 1. 253); in which c being the number of per cents by volume indicated by the alcohometer in any spirit at the tempera- ture t; x, the true percentage by volume of abso- lute alcohol at 15°, which is contained in the spirit in.question, is found by the equation x = c :p 0.4 t. The temperature t is taken as positive above and as negative below 15°. Tables indicating the amount of water required in order to reduce strong alcohol to any required degree have also been given by Gay-Lussac. (Ilandworterbuch der Chem., 1. 257, et seq.) A Mixture of Alcohol and Water Containing per cent, by volume, of abso- T„ „, _.._ . lute alcohol of 0.7939 sp. gr. at 15.56° (the sp.gr. of water at 4.35° being equal to 1.000). 0 0.9991 0 1 0.9976 15 2 0.9961 15 3 0.9947 14 4 0.9933 14 5 0.9919 14 6 0.9906 13 7 0.9893 13 8 0.9881 12 9 0.9869 12 10 0.9857 12 11 0.9845 12 12 0.9834 11 13 0.9823 11 14 0.9812 11 15 0.9802 10 16 0.9791 11 17 0.9781 10 18 0.9771 10 19 0.9761 10 Is of sp. gr. at 15.56° (=60°F.) tween the sp. grs.](https://iiif.wellcomecollection.org/image/b21157091_0033.jp2/full/800%2C/0/default.jpg)