A manual of physics, theoretical and practical : for medical students / by Hugh C. H. Candy.
- Candy, Hugh Charles Herbert, 1859-1935
- Date:
- 1918
Licence: In copyright
Credit: A manual of physics, theoretical and practical : for medical students / by Hugh C. H. Candy. Source: Wellcome Collection.
116/470 (page 102)
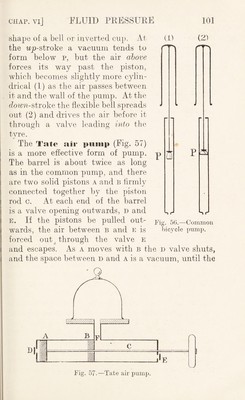
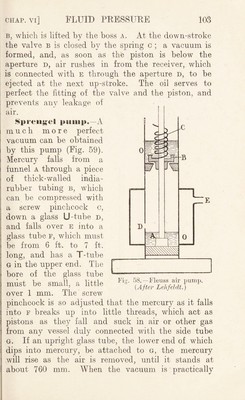
![piston A passes the hole f leading to the receiver, when air rushes in from the bell-jar, to be forced out at the valve d when the pistons are pushed inwards. A similar action takes place with piston b and the space B E. Ex. : If V be the volume of the bell jar and v the volume of air removed by the pump at each stroke, it is clear that a volume P-of air expands to E-f p, and therefore becomes rarefied. If the original density p, is reduced to we have k X P„ = {k + v) X Pi ;■ > ’ ]/ ^and, therefore, pi = p„ x — V V Similarly, the density 'p2, after a second stroke, and finally, after ?i strokes, In the pumps described above tlie valves are opened by the compressed air, and when the air is much rarefied it has not elastic force enough to open the valve, and the pump ceases to act. As a consequence such pumps cannot reduce the air pressure below 1 mm. or 2 mm.—i.e. if they are connected with a vertical glass tube, the lower end of which dips into mercury, they cannot raise the mercury to more than 758 or 759 mm. A. mn?h better vacuum can be obtained by using a valve we rked mechanically, as in the Fieiiss i>iiiiip. In this (Fig. 58) the piston has a boss a, which lifts the valve b mechanically, in the up-stroke. The valve is closed by the spiral spring c. Both the valve and the piston have a layer of oil o o. In the up-stroke, as soon as the piston passes the aperture D, the air above the piston is forced out at the valve](https://iiif.wellcomecollection.org/image/b29927973_0116.jp2/full/800%2C/0/default.jpg)