A manual of physics, theoretical and practical : for medical students / by Hugh C. H. Candy.
- Candy, Hugh Charles Herbert, 1859-1935
- Date:
- 1918
Licence: In copyright
Credit: A manual of physics, theoretical and practical : for medical students / by Hugh C. H. Candy. Source: Wellcome Collection.
203/470 (page 189)
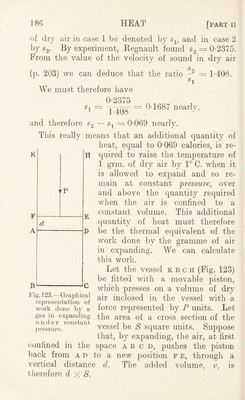
![formed by the combustion of a chemical compound and the heats of combustion of its constituent elements, we can calculate the heat of formation of the compound. Ex. : Marsh gas, methane, OH4, when burnt forms CO 2 and 2H2O : Heat of formation of CO2 • . . . — 94,300 „ „ „ 2H2O . . . 136,600 230,900 Heat of combustion of a molecular weight of CH4 = 213,800 and 230,900 - 213,800 = 17,100. This must be the heat spent in separating the compound GH4 into its elements, previous to their combustion. In other words, 17,100 calories were evolved when 12 grm. of carbon combined with 4 grm. of hydrogen to form 16 grm. of marsh gas. • Heat of combustion of food.—-The heat of form¬ ation of more complex bodies such as the fat, starch, and protein included in an ordinary mixed diet is not known. The heat of combustion has been measured, and is usually stated in large Calories [1 large Calorie (Cal.) = 1,000 small calories (cals.)]. By complete combustion— 1 grm. of protein evolves about .. 5-65 Calories 1 „ „ fat „ „ .. 9*4 1 ,, ,, carbohydrates evolves about 4T5 ,, It must, however, be remembered that the full equivalent of this heat of combustion is not obtained by the consumer when these substances are consumed in human diet. The process of combustion in the body is not carried to the uttermost limit. The food is not burnt to an ash as in artificial combustion. The solids of the fseces and the urine still possess some unused heat of combustion. This must be valued and deducted from’ the above numbers if we](https://iiif.wellcomecollection.org/image/b29927973_0203.jp2/full/800%2C/0/default.jpg)