A hand-book of industrial organic chemistry : adapted for the use of manufacturers, chemists, and all interested in the utilization of organic materials in the industrial arts / by Samuel P. Sadtler.
- Date:
- 1895
Licence: Public Domain Mark
Credit: A hand-book of industrial organic chemistry : adapted for the use of manufacturers, chemists, and all interested in the utilization of organic materials in the industrial arts / by Samuel P. Sadtler. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
43/556 (page 33)
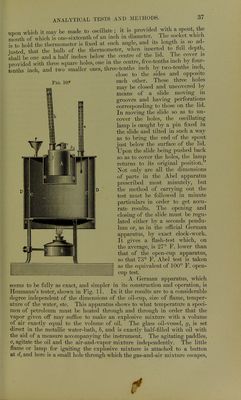
![may remain in them. The fire-test of the solar oil is generally above 100° C, and they are in general both cheap and excellent burning oils. Parqffine Oil.—The paraffine itself has been described under a previous heading. The expressed paraffine oil is used considerably as a lubricating oil, but is of greatest importance for gas-making. The gas from this paraffine oil is especially rich in illuminating hydrocarbons and is free from the ordinary impurities of coal-gas. It is extensively manufactured in Germany, in the Hirzel and Pintsch forms of apparatus, and in England by the Pintsch, Keith, and Alexander & Patterson processes, and compressed for use in railway carriages, etc. Its characters will be referred to more especially under the heading of illuminating gases. 5. Vaseline.—This product (petrolatum of the United States Phar- macopoeia and unguentum parajini of the German Pharmacopoeia) may be obtained from several of the raw materials already mentioned. In the United States, as the name petrolatum indicates, it is a petroleum product and may be called natural vaseline, as it is merely a purified preparation of naturally existing petroleum constituents ; in Germany, and elsewhere in Europe, it is either extracted from other sources (pomade ozokerine), or, as the name unguentum paraffini indicates, it is an artificial vaseline made by the solution of paraffine in paraffine oil. American vaseline, as made by the Chesebrough Company and others, is gotten by taking a vacuum residuum (see p. 26) and, without any treatment with sulphuric acid or other chemicals, simply filtering it through heated bone-black. In this way the amorphous character of the hydrocarbons is not changed and no crys- talline paraffine is produced, as would be the case if it were a distillation product, and, moreover, no traces of sulphonic acids can remain from the acid treatment to interfere with its use as a basis of medicinal ointments. The petrolatum of the United States Pharmacopoeia may be either a soft variety, melting at 40° C. (104° F.), or a firmer variety, melting at 51° C. (125° F.). The German vaseline, or unguentum parqfini, is made by taking one part of ceresine (parajinum solidum) and dissolving it in three parts of a paraffine shale oil, known as vaseline oil {para]ffinum-liqmdum). This artificial vaseline, as Engler and Bohm have shown,* easily becomes granular on chilling, and shows its composite nature moreover by readily separating on distillation into ceresine and oil. The natural vaseline has greater homo- geneity and is more viscous, although at high temperatures it seems to absorb more oxygen then the artificial preparation. At temperatures not exceeding 30° C. the oxygen absorption seems to be practically nothing in either case. Vaseline is largely used in pharmacy and medical practice as a basis of ointments and pomades. IV. Analytical Tests and Methods. 1. For Natural Gas.—These are the methods employed for the analysis of all varieties of illuminating gas, and will be referred to under that headmg. (See p. 382.) 2. For Petroleum.—For liquids in general, the two constants most characteristic are specific gravity and boiling-point. For commercial petroleum products, which are, of course, mixtures of hydrocarbons, the second becomes of only secondary importance, while, with reference to tneir uses as illuminants, the element of safety comes into consideration, so * Dingier, Polytech. Journal, 262, p. 468. 3](https://iiif.wellcomecollection.org/image/b21906269_0043.jp2/full/800%2C/0/default.jpg)