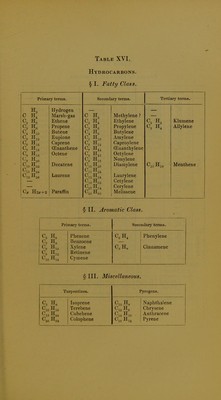
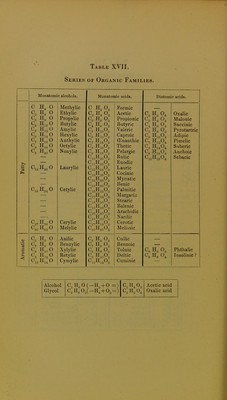
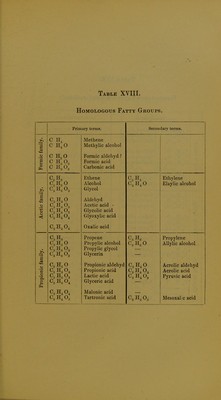
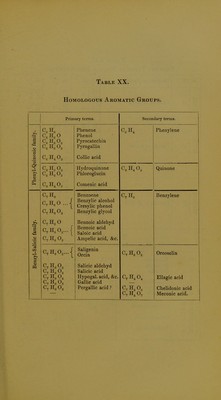
Licence: Public Domain Mark
Credit: Tables of chemical formulae / arranged by William Odling. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
18/18
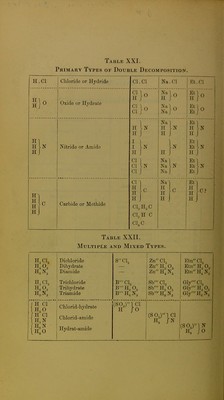
![Primary Types of Double Decomposition. H.Cl Chloride or Hydride CI. CI Na.Cl Et.Cl wxiQe or xiyuraie i'}o H I Nitride or Amide H H i, CI 1 CI CI. 1 Na H H. Na] Na Na. I 1 Etl H Et Et Et] Et Et. I I- HI H H H C Carbide or Methide CI 1 H H H . CI, CI3 CI4 C H,C H C C Na~l H H H ^C i Et! H H H ^C? Table XXII. Multiple and Mixed Types, H,C1, H4O, Hp,N, Dichloride Dihydrate Diamide SC1, Zn CI2 Zn H„ O2 ZnH^N2 EtnCL EtnH,0, EtnH^N2' H3CI3 He03 H9N3 Trichloride Trihydrate Triamide B'CL b'b:.,o, B'HeN3 SVClg Sb' H3 O3 Sb'H6N3 Gly'Cl3 Gly' H3 O3 Gl/HeN3 J H CI JH CI IH3N iHaN 1h,o Chlorid-hydrate Chlorid-amide Hydrat-amide (S0,)1 CI H /O (S0,)-1C1 H, In H3 jo](https://iiif.wellcomecollection.org/image/b22299452_0018.jp2/full/800%2C/0/default.jpg)