Spectrum analysis in its application to terrestrial substances, and the physical constitution of the heavenly bodies / familiarly explained by H. Schellen ; translated from the second enlarged and revised German edition by Jane and Caroline Lassell ; edited with notes by William Huggins ; with numerous woodcuts and coloured plates, and Ångström's and Kirchhoff's maps.
- Heinrich Schellen
- Date:
- 1872
Licence: Public Domain Mark
Credit: Spectrum analysis in its application to terrestrial substances, and the physical constitution of the heavenly bodies / familiarly explained by H. Schellen ; translated from the second enlarged and revised German edition by Jane and Caroline Lassell ; edited with notes by William Huggins ; with numerous woodcuts and coloured plates, and Ångström's and Kirchhoff's maps. Source: Wellcome Collection.
48/748 (page 18)
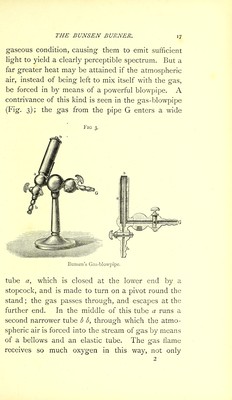
![round the edge, but also in the centre, that an enormous quantity of heat is generated by the complete combustion of the hydrogen and carbon. Over the escape end, a tube slides up and down, and partly by this means, and partly by the cocks, the degree of heat in the flame can be regulated at will. The greater the quantity of gas which can be burnt in a given space, and the greater the energy and the rapidity of the combustion, the greater also will be the amount of heat evolved. For this reason, in the great laboratories, the atmospheric air is forced by a special air-pump into a strong iron receiver of the capacity of several quarts, where it is subjected to a pressure of one and a half or two atmospheres. If this compressed air be allowed to escape along with a copious stream of gas from a common tube, in the same manner as we have just described, the flame becomes one of such intense heat, owing to the rapid and complete com- bustion of so large a quantity of carburetted hy- drogen, that it has power to melt in a few minutes considerable quantities of the least fusible metals, as, for example, a couple of pounds of platinum.# 4. The Magnesium Light. There are some substances, such as potassium, sodium, etc., which have so great an affinity for oxygen that they wrest it even out of its most inti-' * [For the melting of platinum, air and hydrogen or oxygen and coal gas should be used.]](https://iiif.wellcomecollection.org/image/b28057892_0048.jp2/full/800%2C/0/default.jpg)