Spectrum analysis in its application to terrestrial substances, and the physical constitution of the heavenly bodies / familiarly explained by H. Schellen ; translated from the second enlarged and revised German edition by Jane and Caroline Lassell ; edited with notes by William Huggins ; with numerous woodcuts and coloured plates, and Ångström's and Kirchhoff's maps.
- Heinrich Schellen
- Date:
- 1872
Licence: Public Domain Mark
Credit: Spectrum analysis in its application to terrestrial substances, and the physical constitution of the heavenly bodies / familiarly explained by H. Schellen ; translated from the second enlarged and revised German edition by Jane and Caroline Lassell ; edited with notes by William Huggins ; with numerous woodcuts and coloured plates, and Ångström's and Kirchhoff's maps. Source: Wellcome Collection.
58/748 (page 28)
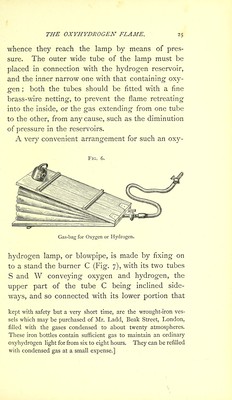
![6. Drummond’s Lime-light. In order to make the oxyhydrogen flame a source of intense light, a cylinder, D (Fig. 7), of well- burnt lime is placed upon the socket of the lamp, and the flame directed against its upper part; it begins at once to glow, and throws out a dazzling light. The oxyhydrogen light, or Drummond’s lime-light as it is sometimes called, after its discoverer, attains a still higher intensity, if a piece of magnesium or zirconia be substituted for the cylinder of lime— an arrangement that has often been adopted in the public illuminations in Paris. While the lime cylin- der slowly consumes in the oxyhydrogen lamp, so that fresh surfaces must be constantly presented to the flame, the piece of zirconia does not waste, and remains unchanged, in spite of the most intense incandescence.* As the heat as well as the light of the oxy- hydrogen flame depends upon the quantity of the burning gases, it is difficult to estimate the tempe- rature with accuracy. In a lamp in which the diameter of the outer tube (hydrogen) is four- * [Huggins found in the spectrum of the light from lime placed in the oxyhydrogen flame, bright lines similar to those which are seen when chloride of calcium is heated in the flame of the Bunsen burner, and which belong probably to volatilized lime, and not to the vapour of calcium. These lines show that a portion of the lime is volatilized by the heat. No lines were seen in the spectrum when zirconia was employed; this earth, therefore, appears to be fixed at the temperature of the oxyhydrogen flame.]](https://iiif.wellcomecollection.org/image/b28057892_0058.jp2/full/800%2C/0/default.jpg)