Spectrum analysis in its application to terrestrial substances, and the physical constitution of the heavenly bodies / familiarly explained by H. Schellen ; translated from the second enlarged and revised German edition by Jane and Caroline Lassell ; edited with notes by William Huggins ; with numerous woodcuts and coloured plates, and Ångström's and Kirchhoff's maps.
- Heinrich Schellen
- Date:
- 1872
Licence: Public Domain Mark
Credit: Spectrum analysis in its application to terrestrial substances, and the physical constitution of the heavenly bodies / familiarly explained by H. Schellen ; translated from the second enlarged and revised German edition by Jane and Caroline Lassell ; edited with notes by William Huggins ; with numerous woodcuts and coloured plates, and Ångström's and Kirchhoff's maps. Source: Wellcome Collection.
59/748 (page 29)
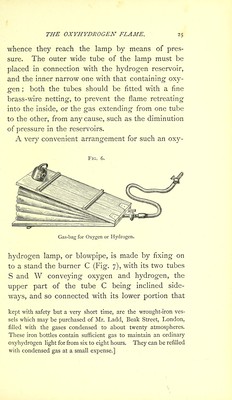
![DRUMMONDS LIME-LIGHT. tenths of an inch, and that of the inner one (oxygen) one-fifth of an inch, the strength of the light is at least equal to that of 180 Stearine candles; the temperature at which platinum melts is about 1,470° C. (2,678° Fahr.); but the heat of this flame under ordinary pressure is estimated by Bunsen to be 2,800° C. (5,070° Fahr.)* As the oxyhydrogen light and the magnesium light are employed in a variety of ways,—not only in public illuminations, but also in theatrical displays, in the exhibition of dissolving views, and in the gas microscope,—so the non-luminous flame renders important service to spectrum analysis on account of its extraordinary heat, in which many sub- stances may be rendered luminous in a state of vapour. The facility with which oxygen gas can now be produced in large quantities, and the possibility of employing ordinary coal gas in place of pure hydro- gen gas combine to render the oxyhydrogen flame a cheap mode of developing an extraordinary de- gree of heat and light, easy and safe to manage, and sufficient in most cases to exhibit, even to a * [Pouillet gives 3,082° F. as the melting point of platinum. By calculations founded upon the amount of heat ascertained by Andrews and others to be emitted during the combustion of a given weight of hydrogen, and the experiments of Regnault upon the specific heat of oxygen, hydrogen, and steam, it has been shown by Bunsen that the temperature of the oxyhydrogen flame cannot exceed 14,580° F., but the actual flame-temperature, as shown by the experiments of Deville and Bunsen, is probably from 4,500° F. to 6,000° F.]](https://iiif.wellcomecollection.org/image/b28057892_0059.jp2/full/800%2C/0/default.jpg)