The principles of inorganic chemistry / by Wilhelm Ostwald ; tr.with the author's sanction by Alexander Findlay.
- Date:
- 1902
Licence: In copyright
Credit: The principles of inorganic chemistry / by Wilhelm Ostwald ; tr.with the author's sanction by Alexander Findlay. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
795/826 page 763
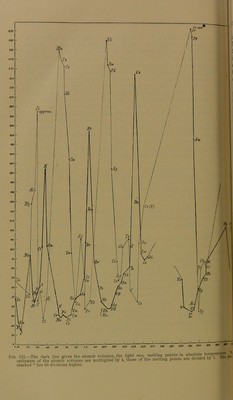
![In this series similar elements always occur at regular intervals. If then the series is divided into a number of sections, so that each section commences with a member of a definite family, it is found that the second, third, and following positions of the sections are also filled by elements corresponding to one another. The table on p. 764 has arisen by dividing the series of the elements, as determined by the values of the combining weights, into such sections; these sections have then been placed one below the other. In this way perpendicular columns are obtained in which similar or related elements stand under one another. The different rows have also been alternately shifted somewhat re- latively to one another. As can be seen, the mutual relation between those elements which are most closely allied to one another thereby receives better expression. Thus, in the column headed 0, we find all the elements of the argon type, which are distinguished by their inability to form chemical compounds. Under I. there are, on the one hand, the monovalent alkali metals, on the other hand the monovalent heavy metals, copper, silver, gold. Under II. there stand the divalent alkaline earth metals, and along with them, the heavy metals of the zinc group. Under III. are the earth metals along with the corresponding heavy metals gallium and indium. Under IY. the tetravalent elements are found. The first repre- sentatives of these have no longer a metallic character, just as the first non-metal appeared in the preceding group in the case of boron; the metals of the titanium group on the one hand, and of the tin group on the other, then follow. Column V. also contains, to begin with, non-metals which can act as trivalent or as pentavalent; in the lower portion there are the corresponding tri- and pentavalent metals. In column VI. are di- and hexavalent elements; the non-metallic character can be followed further down the column. Column VII. contains the typical non-metals, the halogens, which i can act, on the one hand, as monovalent, on the other hand, as heptavalent. Finally, the last column contains the two families of the iron metals and the platinum metals, which do not quite fall into line with the rest of the system. In all these columns the general rule can be observed that the basic properties (i.e. the tendency to form cations) increases with increasing combining weight; the power of forming anions, however, decreases. As can be seen, the table is not complete, but contains many ] positions unfilled. It cannot be otherwise, for there is no justification 1 for the assumption that all existing elements have already been dis-](https://iiif.wellcomecollection.org/image/b21965250_0795.jp2/full/800%2C/0/default.jpg)