Instructions for the chemical analysis of organic bodies / by Justus Liebig ; translated from the German, by William Gregory.
- Justus von Liebig
- Date:
- 1839
Licence: Public Domain Mark
Credit: Instructions for the chemical analysis of organic bodies / by Justus Liebig ; translated from the German, by William Gregory. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
16/66 (page 12)
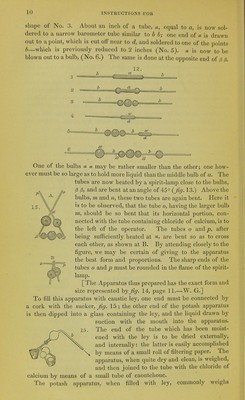
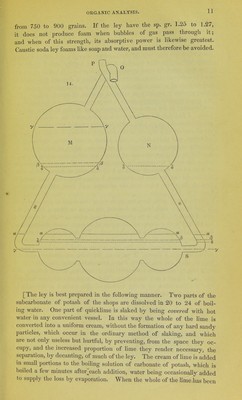
![added, the mixture is boiled for a short time longer, and is then allowed to cool in the pan or goblet, carefully closed with its lid. After 12 hours, nearly the whole of the ley may be decanted perfectly clear, and quite caustic, especially if the vessel has been nearly full. The carbonate of lime, when this process is followed exactly, is sandy, and occupies a very small bulk. The clear liquid is now to be rapidly boiled down in a clean iron vessel, till small crystals begin to separate. It is then allowed to cool in a stoppered bottle of green glass, when it deposits the whole of the sulphate of potash originally present in the subcarbonate; that salt being absolutely insoluble in a strong solution of caustic potash. For the above essential improvements in the preparation of caustic potash, we are indebted to the author of this treatise and to Dr. F. Mohr of Cob- lentz. I find that the solution which has deposited the sulphate of pot- ash possesses the sp. gr. 1.25, and is perfectly adapted for organic analysis. As all contact with organic matter has been avoided, it is also in general colourless, and yields solid caustic potash almost white, containing no impurity except a little chloride of potassium; which of course may be avoided by using genuine salt of tartar; but which does not in the least affect the use of the potash for most purposes. The necessity for using at least 10 or 12 parts of water to 1 of carbonate of potash arises from the curious fact, noticed by Professor Liebig, that when less water is present, the potash takes back the carbonic acid from the carbonate of lime.—W. G.] The tubes of caoutchouc are made out of thin sheets of that substance. A portion, 1^ inch long, is doubled up so as to form a tube of the size of those which it is to connect. About a line in width is now to be cut oflP with a very clean and sharp pair of scissors, along the length of the caoutchouc where the two sides meet. We thus obtain two smooth cut surfaces, which, if pressed together by the thumb nails, adhere so as to form a perfect junction. The tube is now pulled lengthways, so as to stretch it, several times. If we touch the fresh cut surfaces with the fingers, they do not cohere where they have been touched. It is right also to moisten the inside of the caoutchouc, before forming the tube, that its sides may not cohere. The caoutchouc tubes are fastened over the glass tubes by strong threads of silk, knotted at the ends, to prevent them from slipping. [It is more effectual to dust the inside of the tube with any fine pow- der, such as flour or starch—removing all that is superfluous—W. G.] The furnace in which the combustion is carried on, is exhibited by fig. 16. It is made of sheet iron, 22 to ^^jgssagr:: T^]^^^^^^ 24 inches long, and 3 inches high. ^j^^ bottom is 3 inches wide, and furnished with apertures which form . a sort of grate; these apertures are 3> narrow slits, running across, at an half inch distance from each other. The sides of the furnace are in- clined outwards so that at the top they are 4^ inches apart. The whole](https://iiif.wellcomecollection.org/image/b22321603_0018.jp2/full/800%2C/0/default.jpg)