Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
249/332 (page 245)
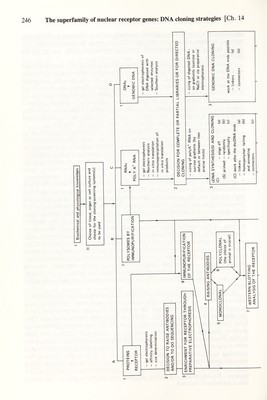
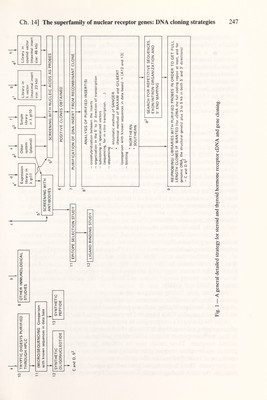
![Ch. 14] The superfamìly of nuclear receptor genes: DNA cloning strategies 245 generation of a large library with less emphasis on the purification of the starting material (estrogen receptor) — here the difficulties are at the screening level. The second strategy was feasible only by using X,gt techniques; today it is by far the preferred one. As depicted in Table 1, the successful isolation of a first cDNA clone was based most often on immunological screenings. Later crosshybridization studies (Northern blots) with mRNA isolated from various species showed that the nucleotide sequence conservation between homologous receptors allowed the screening of cDNA hbraries with nucleic acid probes, thus avoiding the more tedious cloning by expression (see Table 1). In the estrogen receptor subfamily, for example, a cloned human receptor cDNA fragment or a synthetic oligonucleotide based on human receptor cDNA sequences was used as a probe to isolate the rat, chicken and Xenopus laevis estrogen receptor cDNAs (see Table 1 and C8\ 9 in Fig. 1). Note that special care is needed when screening a >-gtll library with a DNA probe which has been purified from a plasmid vector after subcloning. If the vector contains pBR322 sequences, the strains Y1088, Y1089 and Y1090 have to be avoided because their use will result in a high background owing to their episome, which is a pBR322- based recombinant. A strain which does not contain an episome should be used for the screening. We use currently any simple hsdR hsdM^ SupE SupF strain. After isolation, positive clones can be amplified in Y1088 as usual for X,gtll recombinants. A similar route (C4^ or 4^, 5^-8\ 9 in Fig. 1) was used to clone the DNA corresponding to the c-erbA protein. This was particularly interesting because it had been noticed that the product of v-erbA contained sequences homologous to the human estrogen and glucocorticoid receptors (see Chapter 15). Therefore, v-erbA derived probes were used to detect c-erbA cDNA in human X,gtlO or chicken ?igtll libraries (see Table 1 and Chapter 17). To identify the ligand of this putative receptor, cDNA containing the open reading frame was expressed in vitro and in vivo (C8^ in Fig. 1). From the molecular weight of the protein bands observed, only one known hormone receptor was a potential candidate but did not belong to the class of steroid receptors: the thyroid hormone receptor. In fact, it was demonstrated by ligand binding of transiently expressed c-erbA protein that it was identical with a thyroid hormone receptor (see Chapter 17). Using a similar approach, a second distinct thyroid receptor was cloned from human testis (see Table 1) and it is likely that further members of the erbA-thyroid hormone receptor family will be identified soon. The cloning of the human mineralocorticoid and retinole acid receptors (Table 1) represents two important strategies in the isolation of more distantly related members of a superfamily of genes. The cloning of the mineralocorticoid receptor was based on the observation that additional bands appeared on Southern blots hybridized and washed under low stringency conditions when using a glucocorticoid receptor fragment corresponding to a conserved amino acid region in the gene family. These bands were not seen under high stringency conditions, indicating the existence of a distantly related gene. A genomic sublibrary was constructed in >,gtlO according to the Southern blotting data Dl-3, 4\ 5^-7, 8^ in Fig. 1) which was screened under low stringency conditions with the glucocorticoid receptor cDNA fragment. The insert of one positive clone was then used to rescreen X,gtlO cDNA libraries. By expression the cDNA obtained was shown to encode the human mineralocorticoid receptor.](https://iiif.wellcomecollection.org/image/b18029310_0250.JP2/full/800%2C/0/default.jpg)