Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
259/332 (page 255)
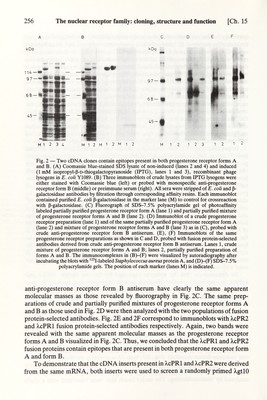
![Ch. 15] The nuclear receptor family: cloning, structure and function 255 synthesized. This sequence, complementary to the mRNA, was designed such that the codon frequency of all known chicken genes was considered and, in ambiguous cases, G was preferred to A so as to allow for G-T mismatches. In this case, a long probe was preferred to a mixture of short oligonucleotides. Further details for the design of oUgonucleotide probes deduced from amino acid sequence data are given in a review by Lathe (1985) on this subject. The progesterone receptor probe ('84- mer') was then used to check Northern blots of polyA* RNA derived from chicken oviducts in different hormonal milieu (DES stimulated, withdrawn, secondary stimulated, laying hen) as well as Southern blots. The results obtained were depressing, since the probe hybridized only to ribosomal RNA, even under stringent conditions. Southern blots showed a smear of hybridization signals. We know now that the sequence chosen was 84% correct (see Fig. 1) but because of the high GC content (86.5%) only ribosomal RNA or the corresponding gene family was detected on Northern or Southern blots. In fact, even when using the correct sequence, we had severe problems with ribosomal RNA crosshybridization on Northern blots. In parallel, a Xgtll cDNA expression library was prepared from size selected (>4 kb) laying hen oviduct poly(A'') RNA and screened with monospecific anti- progesterone receptor form A or form В antibodies. For this the antisera were obtained as described in Chapters 3 and 10 and passed over affinity columns to which homogeneous form A or form В receptor had been covalently coupled. Additio¬ nally, the eluted monospecific antibodies were cleared of dinú-Escherichia coli protein antibodies either by affinity chromatography using E. coli lysate or by absoфtion to E. coli proteins bound to nitrocellulose filters. Screening of 10^ colonies with the antibodies gave two clones that were positive after amplification and rescreening. The ß-galactosidase fusion proteins of the two clones, after induction with isopropyl-ß-D-thiogalactopyranoside (IPTG), had molecular weights of 125 and 130 kDa (Rg, 2A, lanes 1 and 3). Inserts of 250 and 390 bp were found in the corresponding cDNA clones. Immunoblots of the IPTG-induced bacterial lysates confirmed that the fusion proteins crossreacted with the monospecific antiserum (Fig. 2B, middle). Interestingly, both the anti-form A and anti-form В antibodies recognized both fusion proteins. As a control, ß-galactosidase did not react with any of the antibodies (Fig. 2B, right). To demonstrate that the epitopes expressed by the two clones, XcPRl and X,cPR2, correspond to epitopes effectively present on progesterone receptor forms A and B, we selected the corresponding antibodies from anti-progesterone receptor form В antiserum by affinity chromatography on resins containing either one of the two covalently bound purified fusion proteins. These antibodies were used as immunoprobes on immunoblots of crude and partially purified progesterone recep¬ tor. Fig. 2C shows the ñuorograph of partially purified preparations of progesterone receptor form A (79 kDa) (lane 1) and a mixture of progesterone receptor forms A and В (109 kDa) (lane 2) crossUnked with tritiated progestin R5020. Fig. 2D demonstrates the crossreactivity of anti-progesterone receptor form В antiserum with progesterone receptor form A. A crude preparation of a mixture of progester¬ one receptor forms A and В (Fig. 2D, lane 1), a partially purified progesterone receptor form A (lane 2), and a partially purified mixture of progesterone receptor forms A and В (lane 3) were separated on 7.5% SDS-polyacrylamide gels and analyzed by immunoblotting. The major bands that were visualized with a crude](https://iiif.wellcomecollection.org/image/b18029310_0260.JP2/full/800%2C/0/default.jpg)