Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
261/332 (page 257)
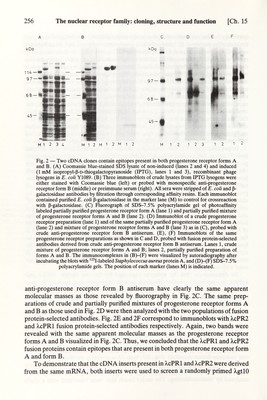
![Ch. 15] The nuclear receptor family: cloning, structure and function 257 library prepared from fractionated laying-hen oviduct poly (A)* RNA. A 1.1 kb cDNA clone, >.cPR3, containing the sequences found in the inserts of both X,cPRl and X,cPR2, was isolated. Sequencing of the A,cPR3 cDNA revealed that in three regions the deduced amino acid sequences are identical to the sequences of three peptides which were isolated from tryptic digests of apparently homogeneous progesterone receptor form В (Simpson et al., 1987; see also Chapter 10). By isolating various overlapping cDNAs as well as the corresponding genomic sequences, the chicken progesterone receptor mRNA was determined as being 4472 nucleotides long. It is important to mention that during the entire cloning work we did not find any evidence for the existence of two different genes, encoding forms A and B. Furthermore, we have not found any indication that form A could be generated from a different RNA by an alternative termination of transcription or alternative sphcing. The cloning of a so-called 'chicken progesterone receptor В antigen' was reported (Zarucki-Schulz et al., 1984). However, it is now known that this cDNA corresponds to a heat-shock protein of a similar molecular weight to the progester¬ one receptor form В (Kulomaa et al., 1986; Sargan et al., 1986; Kleinsek et al., 1988). The same group reported subsequently the cloning of a chicken progesterone receptor cDNA which is apparently identical to the one obtained in this laboratory (Conneely et al., 1986). С. Human glucocorticoid receptor The strategy chosen in our laboratory for the cloning of the cDNA corresponding to the human glucocorticoid receptor was based on the crossreactivity of antibodies which had been raised against the rat glucocorticoid receptor with the human glucocorticoid receptor from MCF-7 breast cancer cells (Govindan et al., 1985). Initially, the MCF-7 receptor was characterized by triamcinolone acetonide mediated photoaffinity labeling and immunoblotting. Monospecific antisera were prepared and used to screen an MCF-7 ^gtll cDNA expression library. Several clones were isolated and, using epitope selection techniques similar to those described above for the progesterone receptor, the identity of the clones with the human glucocorticoid receptor gene derived cDNA was demonstrated. Using polysome immunoadsoфtion techniques (Miesfeld et al., 1984) or approaches basically similar to those described above (Weinberger et al., 1985a), two other groups succeeded in cloning the rat and the human glucocorticoid receptor. Further details may be found in the original literature as well as in the previous chapter by J. M. Jeltsch on cloning strategies. III. STRUCTURE A. Human estrogen receptor mRNA The sequencing of several overlapping MCF-7 estrogen receptor cDNA clones showed that the corresponding mRNA is 6322 nucleotides in length (Green et al., 1986; Fig. 3). The major open reading frame of 1785 nucleotides encodes a 66182 Da protein. The sequence of the 5' end of the estrogen receptor mRNA was determined from a genomic clone isolated from a human library, and the transcription start site](https://iiif.wellcomecollection.org/image/b18029310_0262.JP2/full/800%2C/0/default.jpg)