Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
267/332 (page 263)
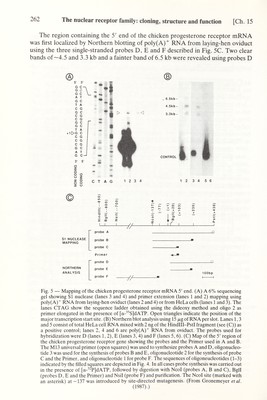
![Ch. 15] The nuclear receptor family: cloning, structure and function 263 and E, whereas no RNA hybridizing with probe F could be detected (Fig. 5B, lanes 2, 4 and 6 respectively). A mixture of HeLa cell total RNA and Hindlll-PstI genomic DNA fragment (Control, see Fig. 5B) was run in parallel in lanes 1,3 and 5. As expected, only the control DNA fragment was revealed in these lanes. These results indicated that the 5' end of the chicken progesterone receptor mRNA was located within a few hundred bases upstream of the Bgll (+20) site (Fig. 5C). The primer extension technique was then used to map more precisely the position of the cap site. A single-stranded ^^P-labeled primer cut at the Egli (+20) site (Primer, see Fig. 5C) was synthesized by elongation of the synthetic oligonucleotide 2 (Fig. 4), using the Hindlll-PstI genomic DNA fragment (Fig. 5C) inserted in M13 as a template. When elongated in the presence of oviduct poly(A)^ RNA with reverse transcriptase and cold dNTPs, this labeled primer yielded a major product (Fig. 5A, lane 2) which mapped at the position of a С in the mRNA coding strand of the DNA (see the sequence ladder of the DNA obtained using the dideoxy method with [^^S] dATP and the oligonucleotide 2 (Fig. 4) as a primer). Some additional bands were visible both upstream and downstream of the main band, suggesting the existence of multiple initiation sites. The 5' end of the mRNA was also mapped with SI nuclease using a ^^P-labeled probe С (Fig. 5C). After hybridization with oviduct poly(A)'' mRNA and SI nuclease digestion, a major SI nuclease-resistant DNA fragment (Fig. 5A, lane 4) migrated at the same position as the reverse transcriptase extended primer (compare lanes 2 and 4). In this case also, several minor bands, possibly corresponding to multiple initiation sites, were visible on the original autoradiogram. Under identical conditions, no primer extension or SI nuclease- resistant products were seen using HeLa cell poly(A)'^ RNA (lanes 1 and 3). Thus the 5' end of the chicken progesterone receptor mRNA is most probably localized in a region centered on the G indicated as +1 in Figs. 1 and 2A and there may be multiple initiation sites. That the 5' end of the chicken progesterone receptor mRNA is located in this region was also suported by SI nuclease mapping experiments (not shown) performed with ^^P-labeled probes A and В (Fig. 2C), which resulted in protected fragments of the expected lengths. All the above results lead us to conclude that the length of chicken progesterone receptor mRNA is very close to 4.5 kb, which is in good agreement with the estimated size of the major poly(A)'^ RNA species revealed on Northern blots with chicken progesterone receptor cDNA probes (see above). The 3.3 kb poly(A)^ mRNA corresponds to a truncated form of the chicken progesterone receptor mRNA which has a 5' end similar to that of the 4.5 kb species but is interrupted in the coding region after amino acid 451. The origin of the 6.5 kb species is unknown but it may correspond to a splicing intermediate. It is important to note that, with the exception of the cDNA clones which correspond to the 3.3 kb mRNA species, we did not find any cDNA clones with a sequence that is not collinear with that of the 4.5 kb mRNA in any of the chicken oviduct cDNA libraries which have been used. In addition, the study of genomic clones did not provide any evidence that the chicken genome may contain different progesterone receptor genes (unpublished results). The progesterone receptor mRNA open reading frame encodes a protein with a deduced molecular weight of 85 743 Da. This is a marked discrepancy with the molecular weight determined from SDS gels. As described below, an unusual poly- GAG stretch, encoding a poly-glutamic acid region in the form В receptor, is responsible for an aberrant migration of this protein on SDS-polyacrylamide gels.](https://iiif.wellcomecollection.org/image/b18029310_0268.JP2/full/800%2C/0/default.jpg)