Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
271/332 (page 267)
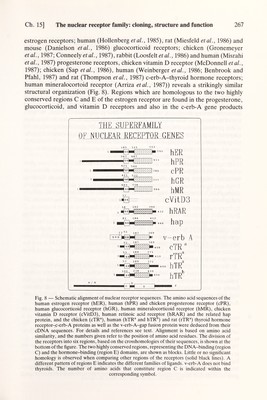
![Ch. 15] The nuclear receptor family: cloning, structure and function 267 estrogen receptors; human (Hollenberg et al., 1985), rat (Miesfeld et al., 1986) and mouse (Danielson et al., 1986) glucocorticoid receptors; chicken (Gronemeyer et al., 1987 ; Conneely et al., 1987), rabbit (Loosfelt et al., 1986) and human (Misrahi et al., 1987) progesterone receptors, chicken vitamin D receptor (McDonnell et al., 1987); chicken (Sap et al., 1986), human (Weinberger et al., 1986; Benbrook and Pfahl, 1987) and rat (Thompson et al., 1987) c-erb-A-thyroid hormone receptors; human mineralocortoid receptor (Arriza et al., 1987)) reveals a strikingly similar structural organization (Fig. 8). Regions which are homologous to the two highly conserved regions С and E of the estrogen receptor are found in the progesterone, glucocorticoid, and vitamin D receptors and also in the c-erb-A gene products THK SUPERFAMil.Y OF NUCLEAR RECEPTOR GF,NES 13 187 369 ^Ii№HZZZZ>i v-erb A 51 187 369 cTR 53 189 371 ттлЯ hTR 1 02 238 4 2 0 К hTR Fig. 8 — Schematic alignment of nuclear receptor sequences. The amino acid sequences of the human estrogen receptor (hER), human (hPR) and chicken progesterone receptor (cPR), human glucocorticoid receptor (hGR), human mineralocorticoid receptor (hMR), chicken vitamin D receptor (cVitD3), human retinoic acid receptor (hRAR) and the related hap protein, and the chicken (cTR®), human (hTR® and hTR ) and rat (rTR®) thyroid hormone receptor-<-erb-A proteins as well as the v-erb-A-gap fusion protein were deduced from their cDNA sequences. For details and references see text. Alignment is based on amino acid similarity, and the numbers given refer to the position of amino acid residues. The division of the receptors into six regions, based on the crosshomologies of their sequences, is shown at the bottom of the figure. The two highly conserved regions, representing the DNA-binding (region C) and the hormone-binding (region E) domains, are shown as blocks. Little or no significant homology is observed when comparing other regions of the receptors (solid black lines). A different pattern of regions E indicates the different families of ligands. v-erb-A does not bind thyroids. The number of amino acids that constitute region С is indicated within the corresponding symbol.](https://iiif.wellcomecollection.org/image/b18029310_0272.JP2/full/800%2C/0/default.jpg)