Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
277/332 (page 273)
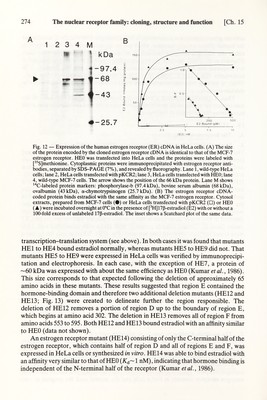
![Ch. 15] The nuclear receptor family: cloning, structure and function 273 activate gene transcription is unknown. It is interesting to note that the С terminus of the human glucocorticoid receptor, which has no apparent sequence homology with either the estrogen receptor or c-erb-A, is essential for binding glucocorticoids (Giguère et al., 1986), whereas the corresponding region of the estrogen receptor is not required for hormone binding (Kumar et ai, 1986). In this respect, it is noteworthy that v-erb-A, which is unable to bind thyroid hormones (Sap et al., 1986), is missing nine amino acids from its С terminus when compared with the chicken c-erb-A. Thus, it appears that region E may form a hydrophobic pocket with some of the non-conserved amino acids providing hgand specificity and invariant amino acids maintaining the structure of the pocket. IV. FUNCTION A. The hormone-binding domain 1. Expression of the human estrogen receptor cDNA in transfected cells The part of the human estrogen receptor cDNA containing the entire coding region (see Fig. 3) was cloned into the eukaryotic expression vector pKCR2 (Breathnach and Harris, 1983) downstream of the SV40 early promoter, producing the human estrogen receptor expression vector HEO (Green et al., 1986; Kumar et al., 1986). НЕО was expressed transiently in HeLa cells, and its ability to direct the synthesis of estrogen receptor was tested using both estrogen receptor monoclonal antibodies and a hormone-binding assay. Fig. 12A shows that the expression of HEO resulted in the appearance of a protein (lane 3, arrowhead) which had the same molecular weight as the estrogen receptor present in MCF-7 cells (lane 4). This protein was not detected in HeLa cells (lane 1) or in HeLa cells transfected with the parent pKCR2 vector (lane 2). Cytosol extracts prepared from HeLa cells transfected with HEO were tested for their ability to bind estradiol (Fig. 12B). Whereas only background binding was detected when using pKCR2 (open squares), significant binding was seen when using HEO (triangles). The protein exhibited an affinity for estradiol {К^= 0.4 пМ) that was very similar to that of the MCF-7 (circles) estrogen receptor (K¿ = 0.5 пМ (Fig. 12В, inset)). Thus, the expression of the estrogen receptor cDNA in HeLa cells yields a protein which, using these criteria, is indistinguishable from the MCF-7 estrogen receptor. The cDNA insert of HEO was also inserted downstream of the T7 promoter of the plasmid vector Bluescribe (Stratagene), allowing large amounts of estrogen receptor mRNA to be synthesized in vitro. Translation of this mRNA in a rabbit reticulocyte lysate produced a 66 kDa protein which bound estradiol with high affinity {K¿ ~ 1 nM) (Kumar et al., 1986; data not shown). 2. The hormone-binding domain is contained in region E A series of contiguous human estrogen receptor deletion mutants (HEl to HE9; Kumar et al., 1986; see Fig. 13) were created using site-directed mutagenesis in order to study the structure-function relationship of the human estrogen receptor. Two approaches were used to localize the estradiol-binding domain of the human estrogen receptor. The first approach involved the transient expression of the human estrogen receptor mutants in HeLa cells and the second used the coupled in vitro](https://iiif.wellcomecollection.org/image/b18029310_0278.JP2/full/800%2C/0/default.jpg)