Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
289/332 (page 285)
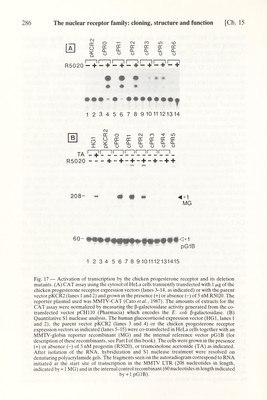
![Ch. 15] The nuclear receptor family: cloning, structure and function 285 Kumar et al., 1987, 1988). It has also been shown that region E of these receptors contains the hormone-binding domain and that this domain can function indepen¬ dently (Kumar et al., 1986). On the basis of these results, we constructed the expression vector cPR3 which contains the C, D and E regions (see Fig. 16). When transfected in HeLa cells grown in an hormone-free medium, cPR3 resulted in the appearance of a truncated chicken progesterone receptor protein which bound progesterone with 'wild type' character¬ istics indistinguishable from those of the protein encoded in cPRO or cPRl (see above; Fig. 3, 'cytopl.', and data not shown). 'Tight' nuclear binding was also observed with the truncated receptor encoded in cPR3 (Fig. 3, 'nucl.', and data not shown). These results suggest very strongly that regions С and E of the chicken progesterone receptor have the same functions as those previously established for the corresponding domains of the estrogen and glucocorticoid receptors (see above). In particular it appears that the highly conserved region E can function as an independent progestin-binding domain because expression of the isolated region E resulted in the appearance of a truncated protein capable of binding progestins (Fig. 16). As expected, no hormone binding could be detected in cells transfected with recombinants in which region E was deleted (cPR4, cPR5 or cPR6, see below and Fig. 16). It has been shown that not only the glucocorticoid receptor, but also the progesterone receptor, can activate initiation of transcription in vivo from the MMTV LTR by interaction with its HRE (Cato et al., 1986,1987). This observation prompted us to investigate whether the 'cloned' chicken progesterone receptor transactivates transcription from the MMTV LTR. cPRO and cPRl were transfected in HeLa cells grown in the presence or absence of R5020, together with the MMTV- CAT reporter gene (Cato et al., 1987). A clear hormone-dependent stimulation of CAT expression was observed with cPRO, cPRl or cPR2 (Fig. 17A): compare lanes 3, 5 and 7 with lanes 4, 6 and 8; see also lanes 1 and 2 which correspond to transfections with the parent expression vector pKCR2. The results shown in Fig. 17A indicate also that the truncated chicken progesterone receptors encoded in cPR3 (lanes 9 and 10) and cPR5 (lanes 11 and 12) had a drastically decreased stimulatory activity which, as expected, was independent of the presence of the hormone in the case of cPR5. No stimulation could be detected when cPR6 (lanes 13 and 14) was used. In order to quantify more rigorously the transcriptional stimulatory activity of the various deletion mutants, RNA was determined by quantitative SI nuclease analyses using an MMTV LTR based reported recombinant in which the CAT sequence was replaced by a promoterless rabbit ß-globin gene (MMTV-globin or MG). In addition, in order to be able to correct for variations in transfection efficiencies, an internal control recombinant (pGlB) containing the rabbit ß-globin gene and its promoter was co-transfected. Using this system, the RNAs initiated at the MMTV LTR start site (-1-1 MG in Fig. 17B) and at the globin start site of pGlB (-Ы pGlB) could be quantitatively determined by scanning the autoradiograms of polyacryl- amide-urea gels, following hybridization with a single stranded ^^P-labeled DNA probe and nuclease SI digestion. The results of a representative transfection experiment in HeLa cells are displayed in Fig. 17B, and the average values obtained by scanning at least three similar autoradiograms are given in Fig. 16, relative to](https://iiif.wellcomecollection.org/image/b18029310_0290.JP2/full/800%2C/0/default.jpg)