Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
291/332 (page 287)
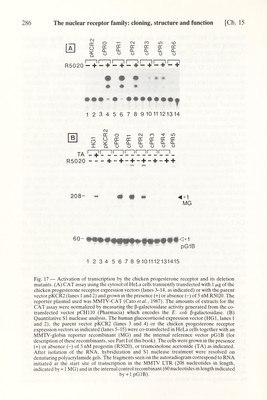
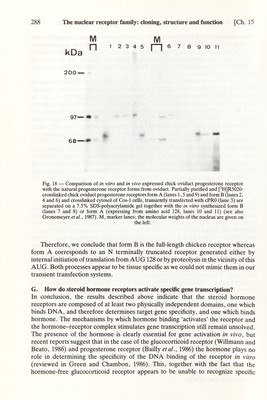
![Ch. 15] The nuclear receptor family: cloning, structure and function 287 those obtained with cPRO (taken as 100%, after correction for transcription from the internal control pGlB). A very strong stimulation (>100-fold) was observed with cPRO in the presence of the progestin R5020. In fact, this stimulation was ~4-fold higher than that obtained under identical conditions (but in the presence of triamcinolone acetonide) with HGl, a pKCR2-based vector expressing the glucocor¬ ticoid receptor (Kumar et al., 1987). The stimulatory activity of cPRl was very close to that of cPRO whereas that of cPR2 which encodes a truncated receptor which may correspond to the 'natural' chicken progesterone receptor form A (see below) was decreased by ~2-fold. In agreement with the results of the CAT assay, deletion of the A-B region to amino acid 405 resulted in a decrease in stimulation of transcription by at least two orders of magnitude (cPR3 in Fig. 17B, lanes 11 and 12). This is particularly striking because the truncated protein encoded in cPR3 bound progesterone with 'wild-type' char¬ acteristics and was 'tightly' bound to the nucleus in the presence of progestins (see above). Finally, very httle (cPR4 and cPR5, Fig. 17B, lanes 13-15) or no (cPR6) stimulation of transcription was observed with the other deletion mutants, as expected from the results with MMTV-CAT. Recently we have constructed mutants in which 35 amino acids were added N terminally to cPR3, as well as further mutants with different C-terminal truncations in region E. The results obtained support the view that both A-B and E are required for efficient activation of the MMTV-HRE by the progesterone receptor. F. Origin of two chicken progesterone receptors On the basis of the results obtained during the cloning of the chicken progesterone receptor cDNA and gene, several possibilities for the origin of two receptor forms with extensive structural and immunological similarities could be excluded. For example, we did not find any evidence for the existence of a second receptor gene or for a cDNA encoding the hormone binding domain which was not collinear with the one described in Fig. 2. Since both forms bind progestins with identical characteris¬ tics and since the hormone-binding domain was localized in the carboxy-terminal region, two possibilities were considered as the most likely ones: (i) internal initiation from the only AUG present in the 5' half of the RNA, or (ii) proteolysis in this region. If internal initiation actually occurred in the chick oviduct, an expression vector encoding only the C-terminal region of AUG 128 (see Fig. 2) should produce a protein of the same length as the natural form A. Fig. 18 shows that in vitro expression of cPRG2 (containing the sequences for amino acid 128 to the carboxy terminus) in fact produces a protein with a molecular weight similar to that of the photoaffinity labeled form A. Additionally, extracts of Cos-1 cells transiently transfected with expression vectors encoding the same progesterone receptor region exhibited a 79 kDa band similar to the chick oviduct receptor form A on immuno- blots (Fig. 19). Although in vitro internal initiation accounts, at least in part, for the production of a 79 kDa protein (Gronemeyer et al., 1987), following transient transfection of Cos-1 cells only traces of form A could be detected. These results were obtained even when expressing a cDNA containing most of the 5'-untranslated region, the ATGTGA motif, and the natural Lozak's sequences which should most accurately reproduce normal translational regulation (Fig. 19, lane 2; see also Gronemeyer et al., 1987).](https://iiif.wellcomecollection.org/image/b18029310_0292.JP2/full/800%2C/0/default.jpg)