Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
293/332 (page 289)
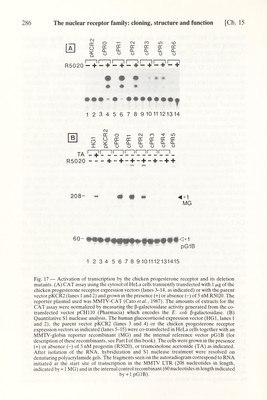
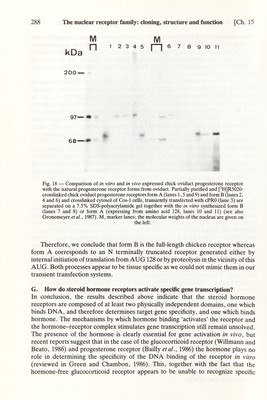
![Ch. 15] The nuclear receptor family: cloning, structure and function 289 12 3 4 к Da 109>- 79^ - m Fig. 19 — Western blot of cytoplasmic extracts of Cos-1 cells transiently transfected with cPRO (lane 2), cPR2 (lane 3) or cPR2 (lane 4). A Western blot of a partially purified chick oviduct progesterone receptor form В preparation, run on the same gel, is shown for comparison (lane 1). The positions of form В (109 kDa) and form A (79kDa), still present in the form В preparation, are indicated. responsive elements of a target gene in vivo (Becker et al., 1986), has led to the speculation that the role of the hormone in vivo is to 'unmask' a pre-formed DNA- binding domain hidden in the unoccupied receptor by an as-yet unknown structure. This might be, for example, a part of the receptor itself or the 90 kDa heat shock protein (Joab et al., 1984; reviewed in Green and Chambón, 1986). If this assump¬ tion is correct, then our own results indicate that the function of the hormone- binding domain cannot simply be to mediate the masking of the DNA binding domain, since mutants where the hormone-binding domain, contained in the C- terminal half of the estradiol receptor, is deleted cannot efficiently activate gene transcription whereas they still recognize efficiently the ERE (see above). Thus, the occupied hormone-binding domain may be necessary for activation, directly or indirectly, of the transcription machinery. This assumption is further supported by experiments in which the transcriptional activating domain of GAL4, a yeast transcription factor, was replaced with the hormone-binding domain of the estrogen receptor, yielding a chimera which is fully active in the transactivation of transcrip¬ tion from an estrogen-responsive element (Webster et al., 1988). The same may not be true for the glucocorticoid receptor where gene transcription can be stimulated efficiently when using some, but not all, truncated glucocorticoid receptors in which the hormone-binding domain has been removed (Godowski et al., 1987; Hollenberg et al., 1987). It cannot be excluded that in these cases the truncation might generate a transcriptional activating domain pre-existing in the receptor in a different confor¬ mation. It is of interest that the estrogen receptor mutants HE15 and HE21 are still able to stimulate gene transcription, even if stimulation is only 5% of that seen with HEO. This suggests that region С together with a small amount of region D (i.e. the difference between HE19 and HE15) contain all that is necessary for DNA binding and at least an element of that which is required for gene activation, but that the](https://iiif.wellcomecollection.org/image/b18029310_0294.JP2/full/800%2C/0/default.jpg)