Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer.
- Date:
- [1988]
Licence: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
Credit: Affinity labelling and cloning of steroid and thyroid hormone receptors / edited by H. Gronemeyer. Source: Wellcome Collection.
317/332 (page 313)
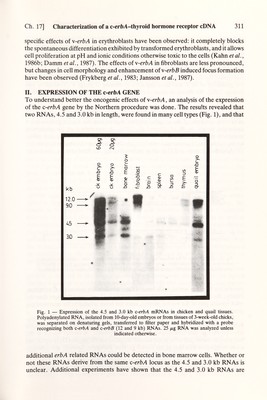
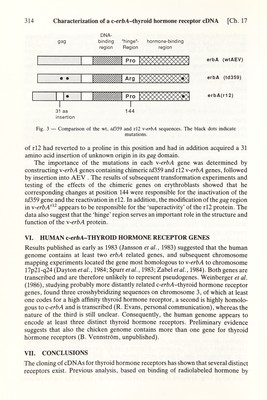
![Ch. 17] Characterization of a с-егЬЛ-thyroid hormone receptor cDNA 313 synthesized c-erbA protein would bind ^^^I-Тз in in vitro binding assays. The resuhs showed that the c-erbA protein bound the hgand with an affinity А:^~0.2-0.3 пМ similar to that of the native thyroid hormone receptor. In addition, the c-erbA protein bound analogues of thyroid hormone with all the characteristics that typify the thyroid hormone receptor (Sap et al., 1986), unambiguously demonstrating that the c-erbA protein is a high affinity nuclear receptor for thyroid hormone. Similar results have been obtained by Weinberger et al. (1986) studying human c-erbA genes. IV. THE \-erbA PROTEIN IS DEFECTIVE The \-erbA protein is defective in binding thyroid hormones (Sap et ai, 1986), presumably as a result of the many mutations it has accumulated. To identify which of the mutation(s) in \-erbA that conferred the inability to bind hormone, a series of recombinants between the viral and cellular genes was constructed utilizing restric¬ tion sites common to both genes. The sites were chosen so that the influence on hormone binding of the C-terminal deletion as well as the amino acid substitutions in \-erbA could be tested. The chimeric genes, cloned into plasmids containing the prokaryotic promoter, were transcribed and translated in vitro, and the resulting proteins tested for binding of ^^^I-T3. The results showed that the 9 amino acid deletion in the С terminus of \-erbA considerably diminished but did not completely abolish ligand binding. In addition, the c-erbA constructs containing the entire putative Ugand binding domain of w-erbA bound no ligand. Taken together, the results so far suggest that both the amino acid substitutions and the C-terminal deletion in \-erbA diminish T3 binding; testing of additional chimeras as well as determination of dissociation constants for ligand binding for all the recombinant proteins are, however, required for a complete evaluation of the effects of the mutations. In addition, an analysis of their properties in vivo could reveal whether the mutations in the putative DNA binding region of the w-erbA protein have altered its specificity for the nucleotide sequences recognized as well as the effects on transcription. In conclusion, the data suggest that the hormone binding site of the c-erbA protein is located in the homologous domain previously shown to be the hgand binding region of steroid receptors (see Chapter 15) and, furthermore, underscore the relatedness between the steroid and thyroid hormone receptors. V. ANALYSIS OF MUTANT v-erbA GENES We have also characterized the \-erbA oncogenes of td359, a transformation- defective mutant of AEV unable to transform erythroblasts, and a revertant rl2, obtained after in vivo passage of the mutant. Biological testing of their s-erbA genes showed that \-erbA'^^^^ is inactive, whereas \-erbA^^^ has an effect on erythroblasts even stronger than that of the wt gene (Damm et al., 1987). Nucleotide sequence analysis showed that the \-erbA protein of td359 contained among other substitutions one amino acid change (proline to arginine) located in the short 'hinge' (Fig. 3) region at position 144 (Damm et al., 1987). The \-erbA protein](https://iiif.wellcomecollection.org/image/b18029310_0318.JP2/full/800%2C/0/default.jpg)