The physiological action of quinoline, isoquinoline and some of there derivatives / by Ralph Stockman.
- Stockman, Ralph.
- Date:
- [1893]
Licence: Public Domain Mark
Credit: The physiological action of quinoline, isoquinoline and some of there derivatives / by Ralph Stockman. Source: Wellcome Collection.
Provider: This material has been provided by The University of Glasgow Library. The original may be consulted at The University of Glasgow Library.
3/8
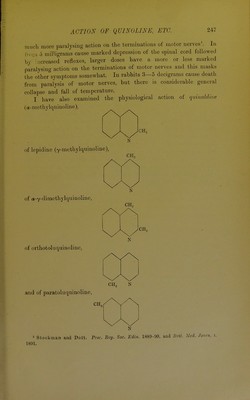
![[From the Journal of Physiology. Vol. XV. No. 3, 1893.] THE PHYSIOLOGICAL ACTION OF QUINOLINE, ISO- QUINOLINE AND SOME OF THEIR DERIVATIVES. By RALPH STOCKMAN, M.D., F.R.C.P.E. {From the College of Physicians' Laboratory, Edinburgh.) In quinoline, isoquinoline, and certain of their immediate derivatives, one has a number of isomeric alkaloids of nearly similar constitution, but having certain of their atoms or radicals differently placed in relation to each other. It occurred to me that it would be of interest to ascertain whether those slight differences in chemical constitution exert any appreciable influence on the physiological action of the bodies in question, more especially as a number of complex alkaloids (such as quinine, cinchonine, -strychnine, morphine) are thought to be derived from quinoline, while recently it has. been proved that others (such as berberine, narcotine, papaverine and hydrastine) are derived from isoquinoline. Quinoline (C9H7N) has the following constitution : II G HC HC H C CH CH 'C II N' but for convenience it is often expressed thus it being understood that CH or C are attached at the unoccupied points of the rings.](https://iiif.wellcomecollection.org/image/b21456744_0003.jp2/full/800%2C/0/default.jpg)