Quantitative chemical analysis : adapted for use in the laboratories of colleges and schools / by Frank Clowes and J. Bernard Coleman.
- Frank Clowes
- Date:
- 1897
Licence: Public Domain Mark
Credit: Quantitative chemical analysis : adapted for use in the laboratories of colleges and schools / by Frank Clowes and J. Bernard Coleman. Source: Wellcome Collection.
581/648
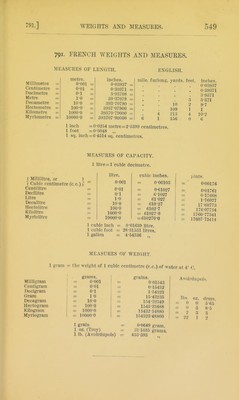
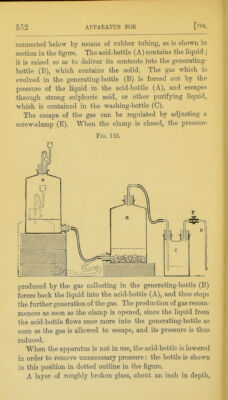
![795.] should be placed upon the bottom of the generating-bottle (B), so as to prevent the solid from remaining in con- tact with acid at the bottom of the bottle when the apparatus is not in use. The bottles are fitted with rubber corks, and these are fastened down by wire to the necks and tubulures of the bottles in order to prevent them from being loosened or displaced. ^ Fig, 116. The Kipp's apparatus (fig. 116) may also be used for generating gases. It is similar in its action to that already described, but the acid vessel is placed above the generating vessel, and the two are connected together rigid- ly when they are fitted up for use. In preparing the following gases, the materials specified below should be used:— Hydrogen; zinc and dilute sul- phuric or hydrochJoric acid. Carbon dioxide; marble and dilute hydrochloric acid. Hydrogen sulphide; ferrous sulphide and dilute hydro- chloric or sulphuric acid. Hydrogen chloride; lumps of rock salt and strong sul- phuric acid. Use of Compressed Gases. 795. Most of the gases which are frequently required for use in the laboratory can now be purchased and stored in the compressed or liquefied condition. Tliey are usually sent out in steel cylinders provided with a screw-valve, and by careful adjustment of this valve, a suitably regulated stream of crag](https://iiif.wellcomecollection.org/image/b21500733_0581.jp2/full/800%2C/0/default.jpg)