On the use of centigrade testing in pharmacy / by John Joseph Griffin.
- John Joseph Griffin
- Date:
- [1851]
Licence: Public Domain Mark
Credit: On the use of centigrade testing in pharmacy / by John Joseph Griffin. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
1/12
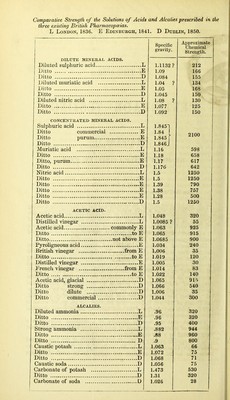
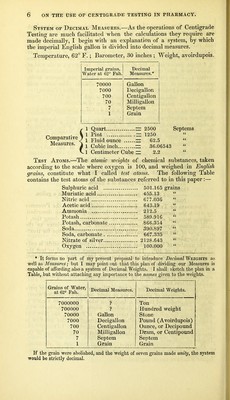
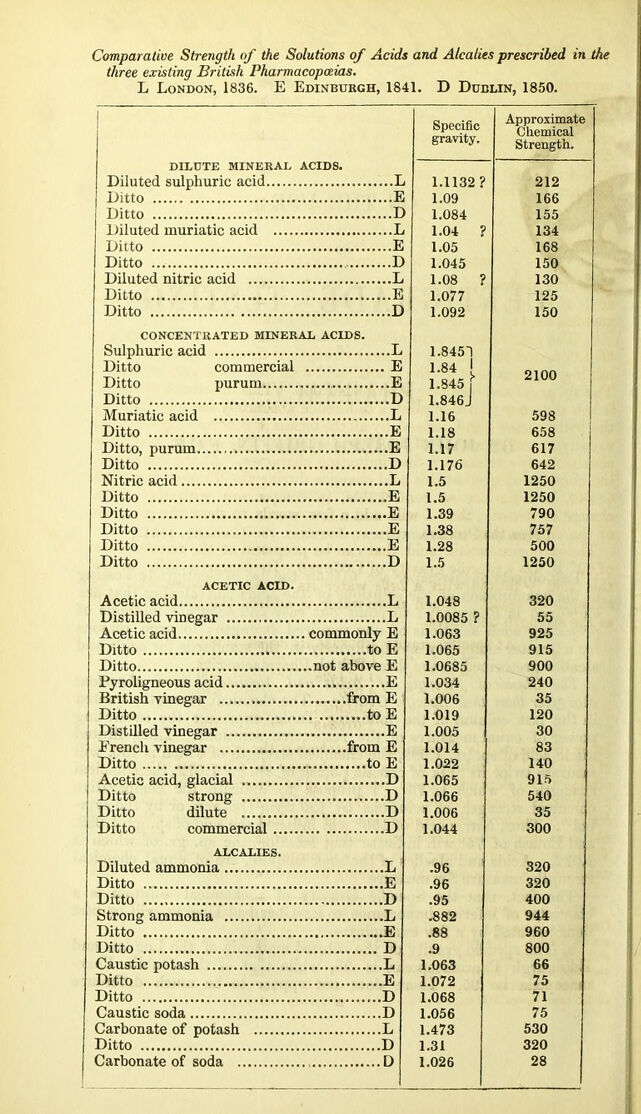
![ON THE USE OF CENTIGRADE TESTING IN PHARMACY. BY JOHN JOSEPH GRIFFIN, F.C.S. [From the Pharmaceutical Journal for January, 1851.] The objects of this paper are, to show the use of Centigrade Testing In the examination of the solutions of the acids and alcalies that are employed in Pharmacy; to point out easy and accurate methods of preparing such solutions of any required strength; to urge reasons for a greater simplicity and uniformity in the standards for acids and alcalies prescribed in the three British Pharmacopoeias. The Chemical Strength of Acids and Alcalies a more important character than their Specific Gravity.—The chemical strength, quoted in the last column of the following Table, expresses the saturating power of equal measures of the respective solutions. Thus, diluted nitric acid of sp. gr. 1.092, the chemical strength of which is 150, will saturate twice its bulk of caustic potash of sp. gr. 1.072, the chemical strength of which is 75. I propose to indicate the comparative chemical strength of solutions by the term Degree, or by the sym- bol °. Thus, Nitric acid of 150° will indicate the acid of sp. gr. 1.092, and Potash of 75° will indicate that solution, the sp. gr. of which is 1.072. Acids and alcalies of the same degree, are of equal chemical strength, measure for measure. The fact, that the Specific Gravity of a chemical solution is a character of inferior importance to its Chemical Degree, will be best demonstrated by a few examples:— a. The specific gravity of the diluted ammonia of the London and Edinburgh Pharmacopoeias is .96, while that of the Dublin Phar- macopoeia is .95. There does not appear to be much difference A](https://iiif.wellcomecollection.org/image/b22376859_0003.jp2/full/800%2C/0/default.jpg)