Laboratory manual of biological chemistry : with supplement / by Otto Folin.
- Folin, Otto, 1867-1934.
- Date:
- [1934], ©1934
Licence: Public Domain Mark
Credit: Laboratory manual of biological chemistry : with supplement / by Otto Folin. Source: Wellcome Collection.
35/388 (page 21)
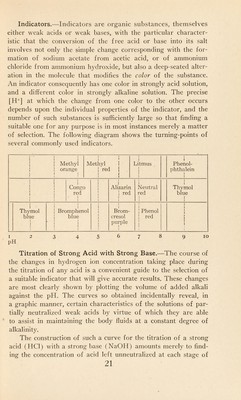
![Indicators.—Indicators are organic substances, themselves either weak acids or weak bases, with the particular character¬ istic that the conversion of the free acid or base into its salt involves not only the simple change corresponding with the for¬ mation of sodium acetate from acetic acid, or of ammonium chloride from ammonium hydroxide, but also a deep-seated alter¬ ation in the molecule that modifies the color of the substance. An indicator consequently has one color in strongly acid solution, and a different color in strongly alkaline solution. The precise [H+] at which the change from one color to the other occurs depends upon the individual properties of the indicator, and the number of such substances is sufficiently large so that finding a suitable one for any purpose is in most instances merely a matter of selection. The following diagram shows the turning-points of several commonly used indicators. 1 * 1 1 ! Methyl 1 orange 1 1 ! i Methyl 1 ! red | 1 f 1 1 1 J Litmus 1 1 1 1 1 T Phenol- phthalein 1 1 l 1 l ! ! 1 1 1 1 1 1 1 l 1 1 Congo red 1 1 1 1 J l 1 1 1 1 1 .1 Alizarin | red 1 1 Meutral •ed 1 Thymol blue 1 1 Thymol blue 1 1 1 1 1 > 1 ! Bromphenol blue 1 1 1 1 1 r 1 1 1 1 1 1 1 1 1 1 1 1 1 Brom- eresol purple 1 Phenol red 1 1 1 1 1 1 1 1 12 3 45678 9 10 pH Titration of Strong Acid with Strong Base.—The course of the changes in hydrogen ion concentration taking place during the titration of any acid is a convenient guide to the selection of a suitable indicator that will give accurate results. These changes are most clearly shown by plotting the volume of added alkali against the pH. The curves so obtained incidentally reveal, in a graphic manner, certain characteristics of the solutions of par¬ tially neutralized weak acids by virtue of which they are able to assist in maintaining the body fluids at a constant degree of alkalinity. The construction of such a curve for the titration of a strong acid (HC1) with a strong base (NaOH) amounts merely to find¬ ing the concentration of acid left unneutralized at each stage of](https://iiif.wellcomecollection.org/image/b29928588_0035.jp2/full/800%2C/0/default.jpg)