Licence: Public Domain Mark
Credit: A system of inorganic chemistry / by William Ramsay. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
649/740 (page 637)
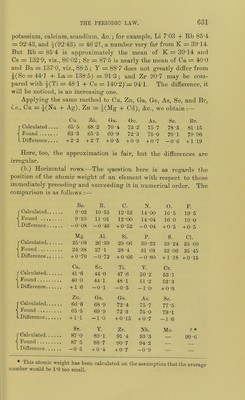
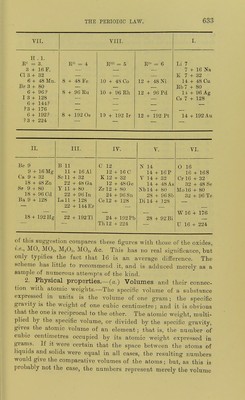
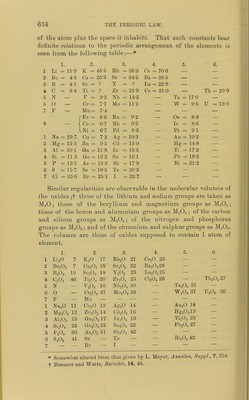
![RjOI Li K Rb Cs — — RCL RO II Be Ca Sr Ba — — RCI2. RjOa III B So Y La Yb — RCI3. RO2I7 C Ti Zr Ce — Tli RCI4. R3O5 7(111).... N V Nb Di Ta — RCI3RCI5. RO3VI (II).... O Or Mo — W U RClo(RCl6'). R,0; VII (I).. .. F Mn — — — — RcI(RClj). fFe fRii. [Oi RO.VIII -^Oo -^Rh \lr LNi LPd [Pfc K-P I N-a Cu — Au — RCL 1^0 II Mg Zn Cd — Hg RCI2. K5O3 III Al Ga In — Tl — RCI3. ROoIV Si Ge Sn - Pb — RCI4. RAV(ni).... P As Sb - Bi - RCl3,RCl5. RO3VI (II) .... S Se Te - - _ RC1„(RC]6). RA-VIII (I)... CI Br I _ _ _ ECL(RC1,). While in L. Meyer's table the alternate elements show analogy with each other, in MendeleefE's table the elements are divided into two classes. We shall consider: 1, the numerical relations of the numbers expressing atomic weights ; 2, some of the physical properties of the elements and their compounds, varying with atomic weight; 3, a comparison of the formula of com- pounds of the elements; and 4, the fulfilment of predic^ tions of the atomic weights and properties of undiscovered elements, and the changes in recognised atomic weights, due to the periodic arrangement. 1. Numerical relations.—These are best seen by reference to Lothar Meyer's arrangement. (a.) Vertical columns.—It is to be noted that the atomic weights of the elements of the Na, Mg, Al, Si, P, S, and CI columns are nearly the mean of those of the nearest elements of the Li, Be, B, C, N, 0, and F columns. Thus, for example, the mean of the atomic weights of Li and K=i(7-03 + 39-14) = 23-08 ; the atomic weight of sodium is 23-06. Calculating in this manner,' we have the following numbers :— Na. Mg. Al. Si. P. S. CL Calculated 23-08 24-5 27-5 30-0 32 6 34 2 37 0 ^o^^^d 23-06 24-4 27-1 28-4 31 0 32-1 35-5 Difference +0-02 +.0-1 4 0-4 +1-6 +1 6 +2-1 +2-5 Here there is a constantly increasing difference. Cu. Zn. Ga. Ge. As. Se. Br Calculated G2 3 63-7 66 4 69-4 72 7 74-1 _ ^ound 63-3 65-5 69-9 72-3 75-0 79-1 Difference -l-o -1-8 -3-5 -2 9 -2-3 -5-0 —](https://iiif.wellcomecollection.org/image/b21980068_0653.jp2/full/800%2C/0/default.jpg)