Volume 1
The elements of materia medica and therapeutics / by Jonathan Pereira.
- Jonathan Pereira
- Date:
- 1849-1853
Licence: Public Domain Mark
Credit: The elements of materia medica and therapeutics / by Jonathan Pereira. Source: Wellcome Collection.
Provider: This material has been provided by King’s College London. The original may be consulted at King’s College London.
906/934 (page 876)
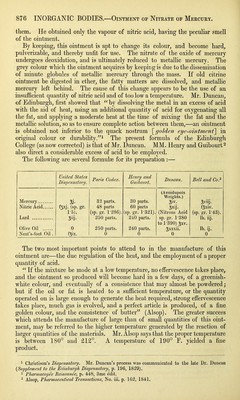
![them. He obtained only the vapour of nitric acid, having the peculiar smell of the ointment. By keeping, this ointment is apt to change its colour, and become hard, pulverizable, and thereby unfit for use. The nitrate of the oxide of mercury undergoes deoxidation, and is ultimately reduced to metallic mercury. The grey colour which the ointment acquires by keeping is due to the dissemination of minute globules of metallic mercury through the mass. If old citrine ointment be digested in ether, the fatty matters are dissolved, and metallic mercury left behind. The cause of this change appears to be the use of an insufficient quantity of nitric acid and of too low a temperature. Mr. Duncan, of Edinburgh, first showed that “ by dissolving the metal in an excess of acid with the aid of heat, using an additional quantity of acid for oxygenating all the fat, and applying a moderate heat at the time of mixing the fat and the metallic solution, so as to ensure complete action between them,—an ointment is obtained not inferior to the quack nostrum [golden eye-ointment] in original colour or durability.”1 The present formula of the Edinburgh College (as now corrected) is that of Mr. Duncan. MM. Henry and Guibourt2 also direct a considerable excess of acid to be employed. The following are several formulae for its preparation :— United States Dispensatory. Paris Codex. Henry and Guibourt. Duncan. Bell and Co.3 Mercury Si- 32 parts. 30 parts. (Avoidupois Weights.) Sviij. Nitric Acid f5xJ- (sp. gr. 48 parts 60 parts oXIj- fsxiv. 1-5). (sp. gr. 1'286). (sp. gr. 1 ’321). (Nitrous Acid (sp. gr. 1-43). Lard Sj- 250 parts. 240 parts. sp. gr. 1'380 lb. iij. Olive Oil 0 250 parts. 240 parts. to 1'390) gxv. ^xxxii. lb. ij. Neat's-foot Oil . fjix. 0 0 0 0 The two most important points to attend to in the manufacture of this ointment are—the due regulation of the heat, and the employment of a proper quantity of acid. “ If the mixture be made at a low temperature, no effervescence takes place, and the ointment so produced will become hard in a few days, of a greenish- white colour, and eventually of a consistence that may almost be powdered; but if the oil or fat is heated to a sufficient temperature, or the quantity operated on is large enough to generate the heat required, strong effervescence takes place, much gas is evolved, and a perfect article is produced, of a fine golden colour, and the consistence of butter” (Alsop). The greater success which attends the manufacture of large than of small quantities of this oint- ment, may be referred to the higher temperature generated by the reaction of larger quantities of the materials. Mr. Alsop says that the proper temperature is between 180° and 212°. A temperature of 190° E. yielded a fine product. 1 Christison’s Dispensatory. Mr. Duncan’s process was communicated to the late Dr. Duncan (Supplement to the Edinburgh Dispensatory, p. 196, 1829). 2 Pharmacopee Baisonnee, p. 448, 3me edit. 3 Alsop, Pharmaceutical Transactions, No. iii. p. 162, 1841.](https://iiif.wellcomecollection.org/image/b21307945_0001_0906.jp2/full/800%2C/0/default.jpg)