Essentials of physiology / by F.A. Bainbridge and J. Acworth Menzies.
- Bainbridge, F. A. (Francis Arthur), 1874-1921.
- Date:
- 1914
Licence: Public Domain Mark
Credit: Essentials of physiology / by F.A. Bainbridge and J. Acworth Menzies. Source: Wellcome Collection.
31/452 (page 19)
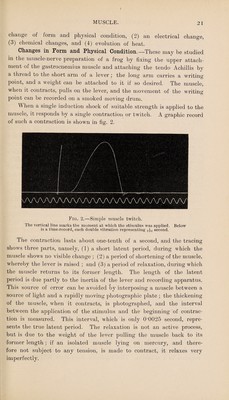
![sarcous element) of each sarcomere is divided into two parts by Hensen’s line, and pervaded with longitudinal canals which are open towards Krause’s membrane; and when the muscle contracts, the clear sub¬ stance at either end of the sarcomere passes into the pores of the sarcous element, so that the sarcomere becomes shorter and thicker. The shortening of the whole muscle is thus regarded as the result of the shortening of its sarcomeres. When a living muscle is examined with polarised light the dim segments are seen to be doubly refracting (anisotropous), while the clear segments are singly refracting (isotropous). In certain animals, such as the rabbit, some of the muscles are pale and others are red in colour. The pale muscles have the structure just described, whereas the red muscle fibres contain more sarcoplasm than the pale ones, and their nuclei are scattered throughout the substance of the fibres; the capillaries also show numerous small saccular dilatations. The red colour is due to the presence of haemo¬ globin in the fibres. These muscles contract more slowly than the pale muscles, but their contraction is more prolonged. In many animals these two varieties of fibre are found together in the same muscle. All muscle fibres are supplied with nerve fibres, some of which are motor and end in the muscle fibres in end-plates, while others are sensory and convey impulses from the muscle to the centra] nervous system. Chemical and Physical Characters of Muscle.—Muscle contains about 75 per cent, of water and 25 per cent, of solid substances, of which proteins form 18 to 20 per cent. The other constituents are a small amount of fat, glycogen (J to 1 per cent.), inosite, and a number of nitrogenous extractives including creatine, xanthine, and hypo- xanthine ; the most important of these is creatine, which forms 0*2 to 0’4 per cent, of the muscle. At a variable period after death the proteins coagulate, the product being called myosin, and the muscles become rigid and opaque; this condition is known as rigor mortis. The nature of the proteins in fresh muscle can be studied if coagulation is delayed by cooling the muscle. The living muscle is minced at a temperature of 0° C., and is then extracted with ice-cold saline solution (0’9 per cent. NaCl) and filtered; the filtrate contains two proteins, namely paramyosinogen and myosinogen. The former is a globulin which coagulates at 47° to 50° C., and constitutes about 20 per cent, of the total protein in muscle. The remaining four-fifths consist of myosinogen, which has the characters of an albumin, though it coagulates at the low temperature of 56° to 60° C. When the solution](https://iiif.wellcomecollection.org/image/b31346960_0031.jp2/full/800%2C/0/default.jpg)