Immunochemistry : the application of the principles of physical chemistry to the study of the biological antibodies / by Svante Arrhenius.
- Svante August Arrhenius
- Date:
- [1907], ©1907
Licence: In copyright
Credit: Immunochemistry : the application of the principles of physical chemistry to the study of the biological antibodies / by Svante Arrhenius. Source: Wellcome Collection.
Provider: This material has been provided by The University of Glasgow Library. The original may be consulted at The University of Glasgow Library.
159/336 (page 141)
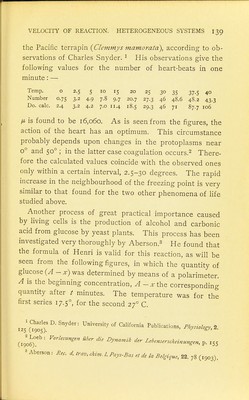
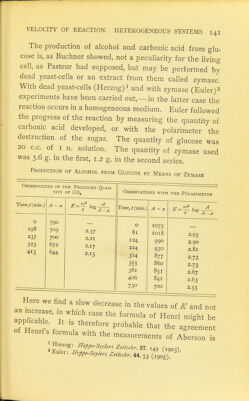
![The production of alcohol and carbonic acid from glu- cose is, as Buchner showed, not a peculiarity for the living cell, as Pasteur had supposed, but may be performed by dead yeast-cells or an extract from them called zymase. With dead yeast-cells (Herzog)i and with zymase (Eu]er)2 experiments have been carried out, —in the latter case the reaction occurs in a homogeneous medium. Euler followed the progress of the reaction by measuring the quantity of carbonic acid developed, or with the polarimeter the destruction of the sugar. The quantity of glucose was 20 c.c. of I n. solution. The quantity of zymase used was 3.6 g. in the first, 1.2 g. in the second series. Production of Alcohol from Glucose by Means of Zymase Observations of the Produced Quan- tity OF CO, Time,i(min.) O 198 237 323 413 790 709 700 672 644 Time, t (min.) 2-37 2.21 2.17 2.15 Observations with the Polarimeter o 81 124 224 324 355 381 406 730 A-. 1075 IO18 990 930 877 860 851 841 700 2-95 2.90 2.81 2-73 2-73 2.67 2.63 2-55 Here we find a slow decrease in the values of K and not an increase, in which case the formula of Henri might be 0^'Hrnri s f ''''^''^ ^^-men^ of Henns formula with the measurements of Aberson is I ^^'■'^■■foPP'-SeyUrs Zeitschr. 37. ,49 (,903). Euler: Hopp.-S.ykrs Zdtschr.ili.. (1905)](https://iiif.wellcomecollection.org/image/b21466312_0161.jp2/full/800%2C/0/default.jpg)