Licence: Attribution 4.0 International (CC BY 4.0)
Credit: Synthesis of cotarnine / by Arthur H. Salway. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
6/14 (page 1211)
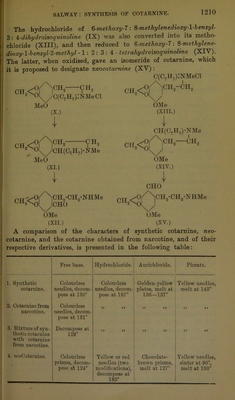
![The synthesis of cotarnine having now been accomplished, that of the alkaloid narcotine has become complete. The stages by which these syntheses have been effected may be represented by the following scheme: Myristicin 4 Myristicinaldehyde 4 3-Methoxy-4 :5-mothylenedioxycinnamic acid 4 0-3-Methoxy-4 :5-methylenedioxyphenylpropionic acid 4 0-3-Methoxy 4 : 5-methylenedioxypheny]propionamide 4 0- 3-Metlioxy-4 :5-mothylenedio^yplienylethylamine 4 Phenylacetyl-8-3-methoxy-4 : 5-methylenedioxyphenylethylamine 4 8-Methoxy-6 : 7-methylencdioxy-l-benzyl-8 : 4-dihydroisoquinoline 4 1- Benzylhydrocotarnine 4 Cotarnine 4 rff-Narcotine. 4 i-Narcotine. Guaiacol Y Guaiacolcarb- oxylic acid > f 5:6-Dimothoxy- trichloro- methylphthal- iae Y 2-Carboxy-3 :4- dimethoxy- mandelic acid Y Meconine Experimental. B-3-M ethoxy-4 : 5-methylenedioxyphenylpropionamide (V, p. 1209). For the preparation of this compound, 100 grams of #-3-methoxy- 4: 5-methylenedioxyphenylpropionic acid,* which had been obtained from myristicin by a series of reactions described in a previous investigation (Trans., 1909, 95, 1208—1210), were dissolved in benzene, and the solution gently warmed with 1 molecule of phos- phorus pentachloride until the reaction was complete. The mixture was then heated on the water-bath under diminished pressure for some time, whereby the solvent, and the phosphoryl chloride which had been formed, were completely removed. The residual crude acid chloride, dissolved in benzene, was gradually added, with vigorous agitation, to an excess of concentrated aqueous ammonia. The * The melting point of 8-8-tnethoxy-4 : 5-methylenedioxyplienylpropionic acid was erroneously given as 124—125° in the previous paper (loc. at.). The correct mcltine Doint of this compound is 99—100°.](https://iiif.wellcomecollection.org/image/b22433090_0008.jp2/full/800%2C/0/default.jpg)