Researches on morphine. Pt. 2 / by S.B. Schryver and Frederic H. Lees.
- Samuel Barnett Schryver
- Date:
- [1901]
Licence: In copyright
Credit: Researches on morphine. Pt. 2 / by S.B. Schryver and Frederic H. Lees. Source: Wellcome Collection.
10/22 (page 570)
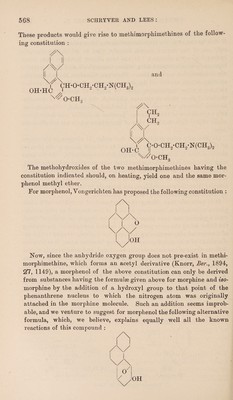
![paper (loc. cit., p. 1035). The method of procedure was as follows. Bromomorphide, in lots of 20 grams, was suspended in 200 c.c. of water contained in a reflux apparatus, and to the slightly warmed mixture just sufficient glacial acetic acid was carefully added to cause the suspended base to dissolve. The solution was vigorously boiled for 3—4 hours, and then evaporated to a syrup on the water-bath. After several successive additions of alcohol and subsequent evapora¬ tion, this syrup formed a crystalline paste, which was drained by the aid of a filter pump : the crystalline salt, after being washed with alcohol until white, was allowed to dry by spreading it on a porous plate. The total amount of alkaloidal salt obtained was only equivalent to somewhat more than 50 per cent, of that theoretically possible. The mother liquor, after further concentration, yielded a very dark coloured syrup, from which no further amount of crystalline product could be obtained. For conversion into base, the salt, in lots of 10—15 grams, was dissolved in 100 c.c. of water contained in a separating funnel; the solution was covered with a layer of pure ether, and rendered alkaline by the addition of 40 c.c. of a saturated solution of sodium carbonate, when the liberated base at first separated and then on shaking went into solution again. On standing, however, it separated in a beauti¬ fully crystalline form. As no further separation of crystalline base occurred after standing for 24 hours, the layer of ether, which was found to contain only a small amount of colouring matter, was separated from the alkaline aqueous liquid containing the crystalline base in suspension. The base was collected by filtration at the pump, washed, first with a little water, then with alcohol, and finally recrys¬ tallised from absolute alcohol, from which it separated in clusters of prismatic needles ; these melted at 247° with slight decomposition, and in methyl alcoholic solution had the specific rotation [a]D = - 166’5°. The product thus obtained (22 grams of base from 45 grams of the alkaloidal salt) was homogeneous and in all respects identical with fsomorphine as obtained from bromomorphide according to the method described in our previous paper. As the amount of crystalline base thus obtained did not account for the whole of the alkaloidal salt employed, it was obvious that the alkaline aqueous filtrate from the fsomorphine still contained a quan¬ tity of base in solution, and in order to obtain this the solution was extracted with hot amyl alcohol until the aqueous liquid was free from organic base, as was shown by acidifying a portion with hydrochloric acid and adding Mayer’s reagent. The amyl alcoholic solution was washed once with a little water, and then extracted four times with dilute hydrochloric acid. The acid aqueous solution of the hydro chloride of the extracted base was evaporated to a low bulk, and after](https://iiif.wellcomecollection.org/image/b30600686_0010.jp2/full/800%2C/0/default.jpg)