Researches on morphine. Pt. 2 / by S.B. Schryver and Frederic H. Lees.
- Samuel Barnett Schryver
- Date:
- [1901]
Licence: In copyright
Credit: Researches on morphine. Pt. 2 / by S.B. Schryver and Frederic H. Lees. Source: Wellcome Collection.
5/22 (page 565)
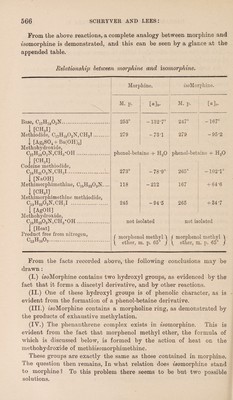
![the fact that fsocodeine methiodide has been obtained by two other methods, namely : (a) By the action of methyl iodide in excess on an alcoholic solution of the sodium derivative of zsomorphine. (b) Codeine, C17H18(0CH3)02N (the methyl ether of morphine), when treated with phosphorus tribromide, yields a crystalline deriva¬ tive, Cl7H17(OCH3)ONBr, which, on treatment with water, decom¬ poses according to the equation : Ci7H]7(OCH3)ONBr + H20 = Cl7H18(0CH3)02N,HBr. By this means, the hydrobromide of a base isomeric with codeine is obtained, which may be designated iso codeine, and this product yields a methiodide identical with that obtained by the two other reactions described above. These reactions prove beyond all doubt the phenol- betaine constitution of fsomorphine methohydroxide, thus clearly de¬ monstrating an analogy with the corresponding morphine derivative. When fsocodeine methiodide is treated with sodium hydroxide, it decomposes according to the equation : Ci6Hi502(OCH3):N(CH3)2I + NaOH = Nal + 'h20 + C16Hu02(OCH3)-N(CH3)2. The base thus obtained is designated in this paper methi-isomorphi- methine, and differs from the base methimorphimethine obtained by an analogous reaction from codeine methiodide in that it is slightly dextro¬ rotatory and melts at 167°, whereas methimorphimethine is strongly laevorotatory and melts at 118°. The methohydroxides of both methi- fsomorphimethine and methimorphimethine, however, yield one and the same nitrogen-free product on heating, namely, morphenol methyl ether, C]5H10O2. These reactions are characteristic of morpholine derivatives, the mechanism of the exhaustive methylation of which may be briefly re¬ presented as follows : * \ /0\ch2 \I/0xCH2 \ /0x'CH2 / \/CH2 /\/ch2 H N(CH3) H N(CH3),I N(CH3)2 H \/°\Ch, ch2 - N(CH3)3 Product free from nitrogen. OH A more detailed rzsumt of the mechanism of these reactions, as interpreted by Knorr, is given in the introduction to our previous paper.](https://iiif.wellcomecollection.org/image/b30600686_0005.jp2/full/800%2C/0/default.jpg)