Researches on morphine. Pt. 2 / by S.B. Schryver and Frederic H. Lees.
- Samuel Barnett Schryver
- Date:
- [1901]
Licence: In copyright
Credit: Researches on morphine. Pt. 2 / by S.B. Schryver and Frederic H. Lees. Source: Wellcome Collection.
6/22 (page 566)
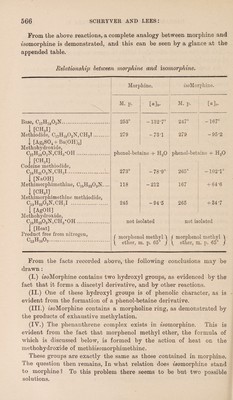
![From the above reactions, a complete analogy between morphine and isomorphine is demonstrated, and this can be seen by a glance at the appended table. Relationship between morphine and isomorphine. Morphine. isoMorphine. Base, Cl7H1903N. I [CHSI] Methiodide, Cl7H1903N,CH3T. 1 [Ag2S04+Ba(0H)J Methohydroxide, C1VH1903N,CH3-0H. , [CH8I] Codeine methiodide, C18H2103N, CH3I. | [NaOH] Methimorphimethine, C19H2303N.... I [CH,I] Methimorphimethine methiodide, C19H2303N,CH3I . I [AgOH] Methohydroxide, C19H2303N,CH3*0H. | [Heat] Product free from nitrogen, ^15^10^2. M. p. [«]»• M. p. [a]o- 253° -132-7° 247° -167° 279 -73-1 279 -95*2 phenol-betaine + H20 phenol-betaine + H20 273° -78-9° 265° -1021° 118 -212 167 + 64‘6 245 -94-5 265 + 34-7 not isolated not isolated / morplienol methyl ) \ ether, m. p. 65° / f morphenol methyl \ \ ether, m. p. 65° J From the facts recorded above, the following conclusions may be drawn: (I.) isoMorphine contains two hydroxyl groups, as evidenced by the fact that it forms a diacetyl derivative, and by other reactions. (ii.) One of these hydroxyl groups is of phenolic character, as is evident from the formation of a phenol-betaine derivative. (III.) 'isoMorphine contains a morpholine ring, as demonstrated by the products of exhaustive methylation. (IY.) The phenanthrene complex exists in tsomorphine. This is evident from the fact that morphenol methyl ether, the formula of which is discussed below, is formed by the action of heat on the methohydroxide of methmomorphimethine. These groups are exactly the same as those contained in morphine. The question then remains, In what relation does fsomorphine stand to morphine? To this problem there seems to be but two possible solutions.](https://iiif.wellcomecollection.org/image/b30600686_0006.jp2/full/800%2C/0/default.jpg)