Nucleic acids : their chemical properties and physiological conduct / by Walter Jones.
- Jones, Walter, 1865-1935
- Date:
- 1920
Licence: In copyright
Credit: Nucleic acids : their chemical properties and physiological conduct / by Walter Jones. Source: Wellcome Collection.
29/168 (page 17)
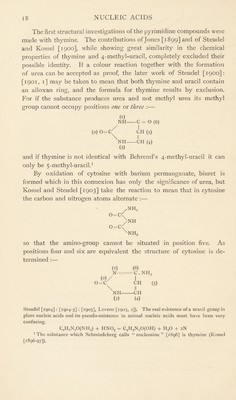
![The Pyrimidine Derivatives of Nucleic Acids. Physiological chemistry is especially concerned with three pyrimi- dine derivatives all of which became known to chemistry for the first time when Kossel and his co-workers found them among the hydro- lytic products of nucleic acids :— (1) Cytosine. (2) Thymine. (3) Uracil. Cytosine is produced by hydrolysis of both animal and plant nucleic acid, but thymine results only from animal nucleic acid and uracil only from plant nucleic acid (Ascoli [1900-1, 2]). Pyrimidine derivatives were given special prominence by the work of Behrend [1885], wbo began his well-known synthesis of uric acid with a substance of this class. By condensation of aceto-acetic ether with urea, uramidocrotonic acid was formed :— 0=C NH, CO . OCoH5 / \ + H H 0=, NFL \ NIL co. oc,h5 I CFI II c. ch3 which by saponification produced 4-methyl-uracil :— NH2 CO . OC2H5 1 NH O II O- 1 1 \ 0 II 0 1 CH O II O \ CH + C,HgOH II \ II \ NH - C . CH., NH c. ch3 From this compound a large number of substances were prepared which Behrend looked upon as derivatives of the hypothetical sub- stance, uracil:— NH- O 0=C' NH- CH II -CH It was from the first apparent that the pyrimidine derivative which Ascoli [1900-1, 2] obtained from yeast nucleic acid has the empirical composition of the hypothetical uracil C4H4N202, and that thymine from thymus nucleic acid is either identical or isomeric with Behrend’s 4-methyl-uracil. Moreover, the transformation of cytosine into uracil by the action of nitrous acid (Kossel and Steudel [1903]) proved that the two substances are corresponding oxy- and amino-compounds.1 1 The same conversion is brought about by violent hydrolysis which accounts for the supposed formation of uracil from animal nucleic acids (Kossel and Steudel [1902-3, 2],](https://iiif.wellcomecollection.org/image/b29807499_0029.jp2/full/800%2C/0/default.jpg)