Nucleic acids : their chemical properties and physiological conduct / by Walter Jones.
- Jones, Walter, 1865-1935
- Date:
- 1920
Licence: In copyright
Credit: Nucleic acids : their chemical properties and physiological conduct / by Walter Jones. Source: Wellcome Collection.
30/168 (page 18)
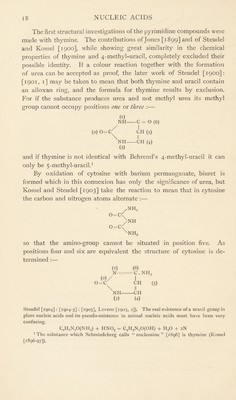
![The first structural investigations of the pyrimidine compounds were made with thymine. The contributions of Jones [1899] and of Steudel and Kossel [1900], while showing great similarity in the chemical properties of thymine and 4-methyl-uracil, completely excluded their possible identity. If a colour reaction together with the formation of urea can be accepted as proof, the later work of Steudel [1900]: [1901, 1] may be taken to mean that both thymine and uracil contain an alloxan ring, and the formula for thymine results by exclusion. For if the substance produces urea and not methyl urea its methyl group cannot occupy positions one or three :—• b) NH C = O (6) / I (2) o=c^ (5) NH CH (4) (3) and if thymine is not identical with Behrend’s 4-methyl-uracil it can only be 5-methyl-uracil.1 By oxidation of cytosine with barium permanganate, biuret is formed which in this connexion has only the significance of urea, but Kossel and Steudel [1903] take the reaction to mean that in cytosine the carbon and nitrogen atoms alternate :— 0=C o=c nh2 NH NH2 so that the amino-group cannot be situated in position five. As positions four and six are equivalent the structure of cytosine is de- termined :— b) (2)/ 0=C (6) C . NH, I CH (5) NH CH (3) (4) Steudel [1904]: [1904-5] : [1905], Levene [1903, 2]). The real existence of a uracil group in plant nucleic acids and its pseudo-existence in animal nucleic acids must have been very confusing. C4H3N20(NH2) + HN02 = C4H3N20(0H) 4- H20 + 2N 1 The substance which Schmiedeberg calls “ nucleosine ” [1896] is thymine (Kossel [1896-97]).](https://iiif.wellcomecollection.org/image/b29807499_0030.jp2/full/800%2C/0/default.jpg)