Chemical technology and analysis of oils, fats and waxes / by Dr. J. Lewkowitsch.
- Julius Lewkowitsch
- Date:
- 1914
Licence: In copyright
Credit: Chemical technology and analysis of oils, fats and waxes / by Dr. J. Lewkowitsch. Source: Wellcome Collection.
Provider: This material has been provided by UCL Library Services. The original may be consulted at UCL (University College London)
239/980 (page 219)
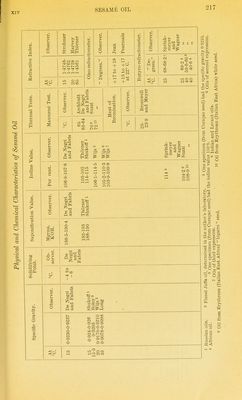
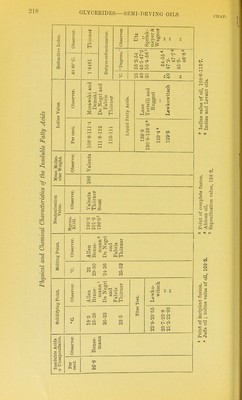
![The amount of unsaponifiable matter in sesame oil varies bom 0-95 per cent to 1-32 per cent. The unsaponifiable matter contams phytosterol, sesamin, and a red oil (see below). The phytosterol was identified by the melting point of the crystals obtamed by crystai- lisincr it seven times fi'om alcohol. Siegfeld ^ thus prepared a phytosterol of the melting point 139-0 -139-2° C. The phytosterol acetate obtamed by Bomer melted at 128°-129° C. Siegfeld prepared crystals meltmg at 130°-131° C. by recrystallising the acetate seven times. Accordmg to Tocher ^ glacial acetic acid extracts from sesame oil two substances— (a) A resinous substance forming long crystalline needles (from alcohol), melting at 118° C. From ultimate analyses and determinations of the molecular weight (by RaouU's method m benzene and acetic acid) the formula CigHigOg was derived. This substance was named sesamin. Sesamin does not give the Baudomn test (see below), but assumes a green and then a bright red colour with nitro-sulphuric acid (the colour reaction given by the U.S. Pharmacopoeia for sesame oil). (b) A thick brown oil (of unknown composition) giving the char- acteristic colour reactions of sesame oil.^ _ • Villavecchia and Fabris ^ found that by extracting the oil itself with either acetic acid or alcohol the chromogenetic substance cannot be whoUy removed. By converting the oil into barium soap, and ex- tracting the latter with alcohol, they isolated from the alcohoUc extract, after evaporating ofi the alcohol, and dissolving the residue m petroleum ether, three substances— 1. An alcohol of the melting point 137° C, and rotatory power [a]^^= -34° 23' (for c = 5-013). This alcohol is doubtless sitosterol; it is not accompanied by stigmasterol {Klamroth 2. Fine crystals having the formula ^'^^ melting at 123° C. {Villavecchia and Fabris) ; according to Bomer and Winter their formula is C33H30O10, and they melt at 122-5° C. The rotatory power is [a]jy = + 68-36 (for c = 24-45) in chloroformic solution. This substance was 'termed sesamin by Villavecchia and Fabris. It is apparently identical with Tocher's sesamin CigHigOg, for theory requires for CjoHigOg, 0 = 68-79 per cent, H = 5-73, and for CnHigOg, 0 = 68-75, H = 6-25, and for C33H30O10, 0 = 67-54 per cent, H = 5-17 per cent. Tocher found (by RaouWs method) the molecular weight of sesamin 311 (in benzene) and 312 (in acetic acid). Villavecchia and Fabns obtained (by RaoulCs method) the molecular weight 350 (in benzene), the formula {'<^i^x2%)2 demanding 384 ; Bomer and Winter's proposed formula C33H30O10 leads to a molecular weight of 586. Sesamin is sparingly soluble in ether and can thus be separated from cholesterol.' 3. A thick, non-crystallisable oil, fi-ee from nitrogen. This oil ' ZeJtx.f. Unters. d. Nahrgs- u. Oenussm., 1904, 585. 2 I'harm. Jnv.rn. and Tran.i., 1891, 639 ; 1893, 700. =< Merkliiig's (./ourn. Soc. Chem. IruL, 1888, 45) statement that it is the glacial acetic acid extract which gives the characteristic colour reaction of sesame oil must tlierclore be corrected. Joiirn. Soc. Chnm. hul., 1894, C9. Klamroth, Inaug. IHsscrl., Municli, 1911. « Annali del Lab. Chim. delle Gab., 1897 (iii.), 22.](https://iiif.wellcomecollection.org/image/b21687560_0239.jp2/full/800%2C/0/default.jpg)