Chemical technology and analysis of oils, fats and waxes / by Dr. J. Lewkowitsch.
- Julius Lewkowitsch
- Date:
- 1914
Licence: In copyright
Credit: Chemical technology and analysis of oils, fats and waxes / by Dr. J. Lewkowitsch. Source: Wellcome Collection.
Provider: This material has been provided by UCL Library Services. The original may be consulted at UCL (University College London)
932/980 (page 900)
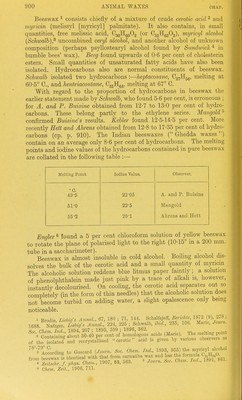
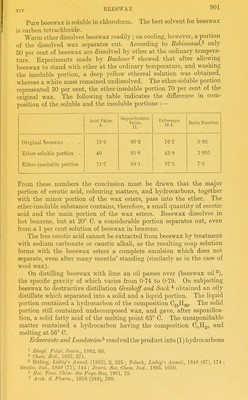
![Beeswax ^ consists chiefly of a mixture of crude cerotic acid ^ and myricin (melissyl [myricyl] palmitate). It also contains, in small quantities, free melissic acid, CgoHgoOg (or Cgj^HggOa), myricyl alcohol {Schwalb),^ uncombined ceryl alcohol, and another alcohol of unknown composition (perhaps psyllostearyl alcohol found by Sundwick * in bumble bees' wax). Berg found upwards of 0-6 per cent of cholesterin esters. SmaU quantities of unsaturated fatty acids have also been isolated. Hydrocarbons also are normal constituents of beeswax. Schwalb isolated two hydrocarbons :—heptacosane, 0271153, melting at 60-5° C, and hentriacontane, O^fi^^, melting at 67° G. With regard to the proportion of hydrocarbons in beeswax the earher statement made by Schwalb, who found 5-6 per cent, is erroneous ; for A. and P. Buisine obtained from 12-7 to 13-0 per cent of hydro- carbons. These belong partly to the ethylene series. Mangold confiimed Buisine's results. Kebler found 12-5-14-5 per cent. More recently Hett and Ahrens obtained from 12-8 to 17-35 per cent of hydro- carbons (cp. p. 910). The Indian beeswaxes ( Ghedda waxes) contain on an average only 8-6 per cent of hydrocarbons. The melting points and iodine values of the hydrocarbons contained in pure beeswax are collated in the following table :— Melting Point Iodine Value. Observer. O. A. and P. Buisine 49-5 22-05 51-0 22-5 Mangold 55-2 20-1 Ahrens and Hett Enghr « found a 5 per cent chloroform solution of yellow beeswax to rotate the plane of polarised light to the right (10-15° in a 200 mm. tube in a saccharimeter). • Beeswax is almost insoluble in cold alcohol. Boihng alcohol dis- solves the bulk of the cerotic acid and a small quantity of myricin , The alcoholic solution reddens blue litmus paper faintly; a solution of phenolphthalein made just pink by a trace of alkaU is, however, instantly decolourised. On cooling, the cerotic acid separates out so completely (in the form of thin needles) that the alcohohc solution does not become turbid on adding water, a sUght opalescence only being noticeable. 1 Brodie Liebir/'s Annul., 67, 180 ; 71, 144. Sclialfejelf, BericlUe 1872 (9) 278 ; 1688 SfegS AnU: 224, 225 ; Schwalb, Mel, 235, 106. Mane, Journ. acids (Marie). The n^eltin, poiut of th?rsoTated°and recrystallised cerotic acid is given by various observers as Acc;rding to Gascard {Journ. Soc. Clwn. Ind., 1893, 955) the myricyl alcohol from beeswax is identical with that from carnauba wax and has the formula C.„H„,0 * ZeZhr. /. phys. Chem., 1907, 53, 563. ' Joum. Soc. Chem. Ind., 1891, 861. « Chem. Zeit., 1906, 711.](https://iiif.wellcomecollection.org/image/b21687560_0932.jp2/full/800%2C/0/default.jpg)