Chemical technology and analysis of oils, fats and waxes / by Dr. J. Lewkowitsch.
- Julius Lewkowitsch
- Date:
- 1914
Licence: In copyright
Credit: Chemical technology and analysis of oils, fats and waxes / by Dr. J. Lewkowitsch. Source: Wellcome Collection.
Provider: This material has been provided by UCL Library Services. The original may be consulted at UCL (University College London)
953/980 (page 921)
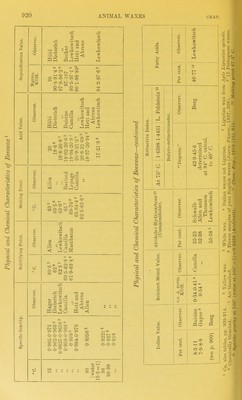
![Berg i who examined a very large number of waxes by Buchner's method (see table, p. 909), points out that the alcoholic solution made up as described above must be allowed to stand 12 hours, as otherwise the results are discordant, and generally too high. Unless a sample be allowed to stand 12 hours, the acids, dissolved by the 80 per cent alcohol are found too high by 20 to 30 per cent. It should be fm-ther pointed out that the detection of stearic acid in bleached waxes, if present only to the extent of 1 to 3 per cent, is very difficult, as chemicaUy bleached waxes may retain small amounts of acid substances The author obtained for genuine yellow beeswax smaller numbers than did Buchner. Thus a Gambia beeswax required for neutrahsation of the fi-ee acids extracted as described above 2-39 c.c. of decmormal alkah ; the isolated acid had the mean molecular weight 406, whereas the same Gambia wax adulterated with varying quantities of stearic acid required of course much higher amounts of decmormal alkah, whilst the mean molecular weights of the isolated fatty acids varied from 274-8 to 298-8. In the latter case appreciable amounts of beeswax cerotic acid had passed into the alcohoHc solution. In doubtful cases it appears advisable to use larger quantities, then to separate the stearic acid as such,^ and to examine it further. It need hardly be pointed out that commercial stearic acid, such as would be used for adulteration, is not pure stearic acid (cp. Vol. III. Chap. XVI.). Buchner * states that on treating 3-6 grms. of beeswax after saponi- fying and evaporating ofi the alcohol with 250 c.c. of boihng water, some beeswaxes yield clear solutions, whereas others (East African, East Indian (Ghedda)) yield turbid solutions or amorphous precipitates. Waxes containing paraffin wax also yield turbid solutions, hence it is necessary to identify the separated substance. This appears to be tantamount to examining the unsaponifiable matter. Detection of Ceresin and Paraffin Faa;.—The examination of beeswax by the saponification process can only reveal the presence of more than 10 or 8 per cent of ceresin and (or) paraffin wax, if no other adulterant be present. If the admixture falls below 5 or even below 8 per cent, the deviations from the normal acid and saponification values become so small that by these two tests alone adulteration cannot be revealed. An excellent preUminary test for the detection of added ceresin and paraffin wax down to 3 per cent has been proposed as a qualitative test by Weinwurm.^ This quaUtative method is based on the fact that a hot aqueous glycerin solution dissolves the unsaponifiable matter of beeswax, whereas ceresin and paraffin wax are insoluble in it. The process is carried out as follows :—Saponify 5 grms. of the sample with 25 c.c. half-normal alcoholic potash (in a 200 c.c. flask), evaporate ofi the 1 Chew. ZeM., 1903, 754. , ^, Thua MedicuH and Wallenstciii {Zeils. f. Uniers. d. NahrijH- u. Wcww.sm,, Jyuz, ]0n-2) showed that tlio acid value of a beeswax bleached with bichronuite and sulphuric acid was as high as 24-7 (cp. also table, p. 913). •'' Cp. Berg, Chem. Zcit., 1908, 779. ■» Zeiinchr.f. OffenU. Clicm., 1911, 225. '- Chan. Zeil.,'U97, 519.](https://iiif.wellcomecollection.org/image/b21687560_0953.jp2/full/800%2C/0/default.jpg)