A treatise on chemistry. Vol. III, The chemistry of the hydrocarbons and their derivatives, or, Organic chemistry. Part I / by H.E. Roscoe & C. Schorlemmer.
- Henry Enfield Roscoe
- Date:
- 1881
Licence: Public Domain Mark
Credit: A treatise on chemistry. Vol. III, The chemistry of the hydrocarbons and their derivatives, or, Organic chemistry. Part I / by H.E. Roscoe & C. Schorlemmer. Source: Wellcome Collection.
Provider: This material has been provided by University of Bristol Library. The original may be consulted at University of Bristol Library.
117/744 (page 99)
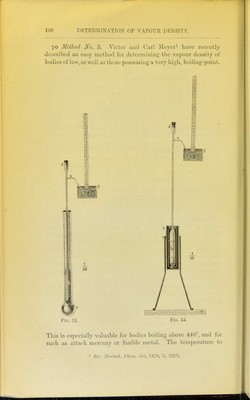
![In the calculation the following data are required : ;S' = Weight of substance. T = Temperature of vapour. t „ „ air. F = Barometric pressure reduced to 0°. p = Excess of joressure in the side-tube. s = Tension of inercury vapour. a = Weight of mercury employed. r = Weight of remaining mercury. q = Weight of mercury contained in the small bottle. The last number is required only in very exact determinations. The calculation is effected by the following formula : 5 X 760 fl + 0-0036657) 13-59 +J) - Si 0-U012y3 (a+c^; ^1 + 0 U0U03U3 [T - t]) - r) (1 + O'OOUiS [T - ij) (1 X 0-OOOlb)' 13'59 is the specific gravity of mercury at 0°. 00000303 is the coefficient of expansion of glass. O'OOOIS ditto of mercury, which above 240° rises to 0-00019. The constants in the above formula are ; 760x13-59 0001293 7988000. The temperature of the vapour does not need to be deter- mined, as the boiling-point of the liquid employed is known. In the case, however, of bodies whose boiling-points approach that of mercury, it is necessary to determine the temperature, as, according to the recent experiments of Naumann, it appears that the boiling-points of liquids which are not miscible undergo considerable depression. Thus he finds that diphenylamine, which boils at a temperature of 310° by itself, boils at 290° when mixed with mercury. The vapour density of benzoic acid was in this way deter- mined in the vapour of diphenylamine with the following results: /S'=0'0603. 2^ = 21 mm. « = 471-7 grams. 290°. r= 66-4 grams. / =--15°-2. !? = 1 gram. s = lQo 7 mm. P= 72G m m. I'^ound. C'alculatcd. Vapoul' density 4*20 4-22.](https://iiif.wellcomecollection.org/image/b2144903x_0117.jp2/full/800%2C/0/default.jpg)