A treatise on chemistry. Vol. III, The chemistry of the hydrocarbons and their derivatives, or, Organic chemistry. Part I / by H.E. Roscoe & C. Schorlemmer.
- Henry Enfield Roscoe
- Date:
- 1881
Licence: Public Domain Mark
Credit: A treatise on chemistry. Vol. III, The chemistry of the hydrocarbons and their derivatives, or, Organic chemistry. Part I / by H.E. Roscoe & C. Schorlemmer. Source: Wellcome Collection.
Provider: This material has been provided by University of Bristol Library. The original may be consulted at University of Bristol Library.
122/744 (page 104)
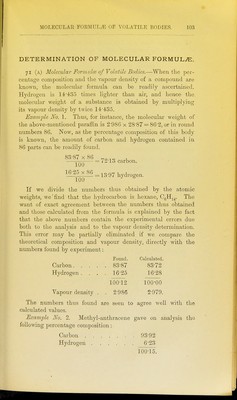
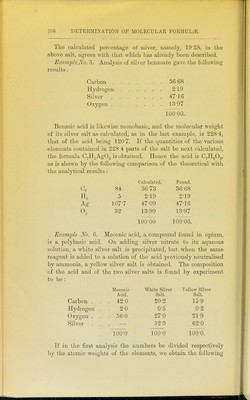
![Its vapour density is G57, and hence its molecular weight is 190, and the amount of carbon and hydrogen contained in 190 parts is: Carbon 178'5 Hydrogen 11 8. These numbers, divided by the approximate atomic Aveights, give: 178-5 12 11-8 = 14-9, = 11-8, showing that methyl anthracene possesses the formula C^sH^o- This corresponds to a theoretical vapour density of 6'63, and a percentage composition of: Carbon 93-75 Hydrogen 6-25 100-00. Example No. 3. As a last example of this kind we may take ethyl propenyl ether, whose vapou]- density determination has been already given. Ultimate analysis gave : Carbon 71*26 Hydrogen 9-55 Oxygen 19-19 100-00. Its molecular weight is 28-87 x 2-895 = 83-6. 83 6 X 71-26 , = o9-60 carbon. 83-6 x 9-55 , YOO = ^ 98 hydrogen. 83-6 X 19-19 100 ^ oxygen. Hence the molecular formula is C^HgO, and this corresponds to : c. 60 71-43 8 9-53 0 16 19-04 100-00.](https://iiif.wellcomecollection.org/image/b2144903x_0122.jp2/full/800%2C/0/default.jpg)