A treatise on chemistry. Vol. III, The chemistry of the hydrocarbons and their derivatives, or, Organic chemistry. Part I / by H.E. Roscoe & C. Schorlemmer.
- Henry Enfield Roscoe
- Date:
- 1881
Licence: Public Domain Mark
Credit: A treatise on chemistry. Vol. III, The chemistry of the hydrocarbons and their derivatives, or, Organic chemistry. Part I / by H.E. Roscoe & C. Schorlemmer. Source: Wellcome Collection.
Provider: This material has been provided by University of Bristol Library. The original may be consulted at University of Bristol Library.
90/744 (page 72)
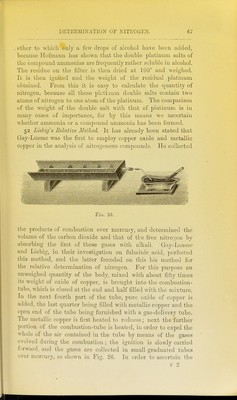
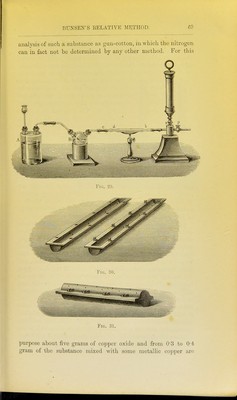
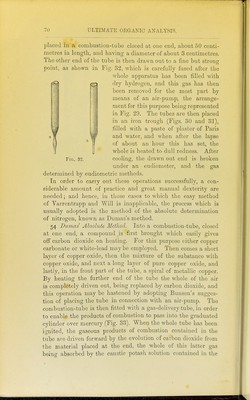
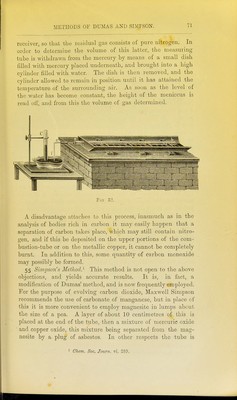
![lllled as described under Dumas' method. The copper spiral should be about 20 centimetres in length, for the purpose not only of decomposing the whole of the oxides of nitrogen which are formed, but also to absorb any excess of oxygen which may come off. The portion of the tube containing the carbonate is first gently heated, and as soon as the evolution of gas becomes rapid, the front part of the tube containing the copper spiral is heated until all the air is driven out of the apparatus, which is readily ascertained by collecting the gases from time to time in a test-tube over mercury and adding a small quantity of caustic potash. As soon as the absorption is complete, the combustion may be proceeded with. The further end of the tube is allowed to cool, and the tube slowly heated from the front towards the back. The gases evolved are collected over mercury in a pear- shaped vessel (Fig. 34) containing caustic potash. As soon as the whole tube is red-hot, and no further evolution of gas is noticed, the gases contained in the tube are swept forward by re-heating the carbonate contained at the closed end. The nitrogen is next transferred to an accurately calibrated eudio- meter, by a process which is rendered sufficiently evident by Fig. 35, and as soon as the caustic potash solution is seen to ascend into the capillary gas-delivery tube, no more mercury is poured in, and thus the exact volume of nitrogen evolved is brought into the eudiometer. Zulkowsky^ has recently described another simpler collecting apparatus, which avoids the use of mercury, and renders it possible to work rapidly. It consists of two tubes of about 58 centimetres in length (a and B, Fig. 36), of which the former is graduated, and serves for collecting and measuring the gas, whilst the latter is open at the bottom, and serves for filling in the caustic potash. Both tubes are held by means of supports (Kjl and k) in a vertical position, and are connected with one another by the caoutchouc tube. Two small tubes (c and c^) are fused on to these tubes. The first of these is connected by means of a caoutchouc tube with the combustion-tube, and can be closed by the ]Dinchcock /. The second small tube serves for letting out the caustic potash, and is also furnished with a pinchcock (c). The small bulbs // contain a few drops of mer- cury, and serve as a safety valve, in order to prevent the caustic potash solution from passing back into the combustion-tube in 1 Liehigs Aimalen, clxxxii. 296.](https://iiif.wellcomecollection.org/image/b2144903x_0090.jp2/full/800%2C/0/default.jpg)