Determination of radicles in carbon compounds / by Dr. H. Meyer. Authorized translation by J. Bishop Tingle.
- Meyer, Hans (Hans Johannes Leopold), 1871-
- Date:
- 1903
Licence: In copyright
Credit: Determination of radicles in carbon compounds / by Dr. H. Meyer. Authorized translation by J. Bishop Tingle. Source: Wellcome Collection.
151/200
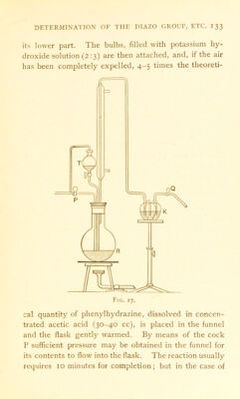
![The results of the analysis are calculated according to the formula N02 = {a — b).0.0007655 gram, where a= the number of cc of iodine solution equivalent to 1 cc of the stannous chloride solution, and £ = the number of cc of iodine solution required in the determination. If it is desired to use the potassium permanganate, 10 cc of the acid liquid, withdrawn as described above, is boiled with ferric chloride, and the ferrous chloride produced is determined in the ordinary manner. (II) Modificd Metltod for Volatile Compounds. Volatile nitro-compounds are weighed in a test-tube about 30 cm by 8 mm, closed with a cork; the cork is removed, and the tube, together with the stannous chloride, placed in a second larger one, 20 cm by 13-15 mm, which is then sealed. The larger tube may be of thin-walled, readily fusible glass, as it will only be subjected to a very slight pressure. The tube is heated in the water-batli during 1-2 hours, and well shaken occasionally; it is then cooled, the contents completely washed into a 100-cc graduated flask, and treated in the manner described in the preceding sec- tion. The use of a sealed tube is sometimes advisable in the case of non-volatile Compounds with which low results may be obtained by heating in the stoppered bottle. Many aromatic nitro-compounds evolve nitrogen quantitatively when treated with phenylhydrazine at or a little above ioo°. (Cf. p. 132.)* R.N02 + 3C6H5NH.NH2 —> R.NH2 -j- ßCgH,. -f- 2H20 -f- 3N2. 1 R. Walter, J. pr. 53 [2], (1896), 437.](https://iiif.wellcomecollection.org/image/b28049123_0151.jp2/full/800%2C/0/default.jpg)