Volume 1
A textbook of human physiology / / translated from [the] 7th German edition by William Stirling.
- Landois, Leonard
- Date:
- 1891
Licence: Public Domain Mark
Credit: A textbook of human physiology / / translated from [the] 7th German edition by William Stirling. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
62/602 (page 22)
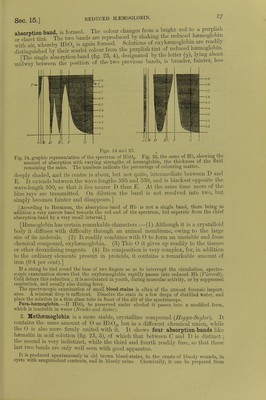
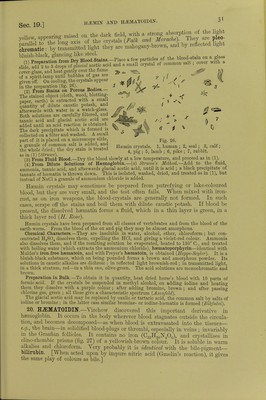
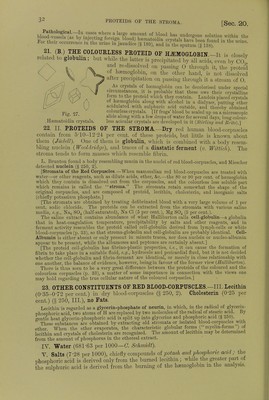
![fiSi oTT' a,Ud h2fSe 5 a.Uf V6ry ,readi'y froni that of the »* and guinea-pig (/Wr) rConeman k i^^ fCOlOU1'ed-Cr};StalS Can ^ °btained from tlle blood of the frog. g Selyal is formed from a single corpuscle enclosing the stroma Crystal* hnvo w,Vfi«, V J *? nucleus of the large corpuscL of fishes, anclin this claTof £t sShS SmaS] C'yStalS °f hffim°globin are ™% ^nd in the prepared EStf*! Dichroism.—Hemoglobin crystals are doubly refractive and pleo-cbromatic ■ they are bluish-red with transmitted light, scarlet-red by reflected light They contain from 3 to 9 per cent, water of crystallisation, and are soluble in water, but more so in dilute alkalies. They are insoluble in alcohol, ether, chloroform, and fats 1 he solutions are dichroic; red in reflected light, and green in trans- mitted light In contact with protoplasmic ceUs, e.g. leucocytes, heemoglobin is destroyed m five days and regenerated again after twelve days (Schwartz). In the act of crystallisation the haemoglobin seems to undergo some internal change. Before it crystallises it does not diffuse like a true colloid, and it also rapidly decomposes hydric peroxide. If it be redissolved after crystallisation, it diffuses, although only to a small extent, but it no longer decomposes hydric peroxide, and is decolorised by it. [The presence of 0 favours crystallisation.] 12. PREPARATION OF HEMOGLOBIN CRYSTALS.-Method of Rollett.-Put defibrinated blood in a platinum capsule placed on a freezing mixture, freeze the blood, and then thaw it ; pour the lake-coloured blood into a plate until it forms a stratum not more than H mm. in thickness and allow it to evaporate slowly in a cool place, when crystals will separate. Method of Hoppe-Seyler. —Mix defibrinated blood with 10 volumes of a 20 per cent, salt solution, and allow it to stand for two days. Remove the clear upper fluid with a pipette, wash the thick deposit of blood-corpuscles with water, and afterwards shake it for a long time with an equal volume of ether, which dissolves the blood-corpuscles. Remove the ether, filter the lake-coloured blood, add to it \ of its volume of cold alcohol (0°), and allow the mixture to stand in the cold for several days. The numerous crystals can be collected on a filter and pressed between folds of blotting-paper. Method of Gscheidlen.—Take defibrinated blood, which has been exposed for twenty-four hours to the air, and keep it in a closed tube of narrow calibre for several days at 37° C. When the blood is spread on glass, the crystals form rapidly. [Vaccine tubes answer very well.] [Method of Stirling and Brito.—It is in many cases sufficient to mix a drop of blood with a few drops of water on a glass slide, and to seal up the preparation. After a few days beautiful crystals are developed. The addition of water to the blood of some animals, such as the rut and the guinea-pig, is rapidly followed by the formation of crystals of haemoglobin. Very large crystals of reduced haemoglobin may be obtained from the stomach of the leech several days after it has sucked blood.] [Crystals of Reduced Haemoglobin may be obtained from human blood ; (1) by the addition to blood of decomposed serum, or of pericardial fluid ; (2) treatment with bile, especially the bile of a cat; (3) agitation with ether ; (4) semi-digestion in the stomach of the leech {Stirling, Bond, Copeman). They may also be obtained as reddish-violet coloured prisms, but green in transmitted light if they are thin, by sealing up some putrefying Hb02 in a tube in an atmosphere of hydrogen (NcncM and Sieber).] 13. QUANTITATIVE ESTIMATION OF H2EM0GL0BIN.—(a) From the Amount of Iron.— As dry (100° C.) haemoglobin contains 042 per cent, of iron, the amount of haemoglobin may be calculated from the amount of iron. If in represents the percentage amount of metallic iron, then the percentage of haemoglobin in blood is = . The procedure is the following:— Calcine a weighed quantity of blood, and exhaust the ash with HC1 to obtain ferric chloride, which is transformed into ferrous chloride. The solution is then titrated with potassic permanganate. (b) Colorimetric Method.—Prepare a dilute watery solution of haemoglobin crystals of a known strength. With this compare an aqueous dilution of the blood to be investigated, by adding water to it until the colour of the test solution is obtained. Of course, the solutions must be compared in vessels with parallel sides and of exactly the same width, so as to give the same thickness of fluid {Hoppe-Seijler). [In the vessel with parallel sides, or hsematinometer, the sides are exactly 1 centimetre apart. Instead of using a standard solution of oxyhemo- globin, a solution of picro-carminate of ammonia may be used (Rajcwsky, Malassez).] (c) By the Spectroscope. —Preyer found that a 08 per cent, watery solution (1 cm. thick), allowed the red, the yellow, and the first strip of green to be seen (fig. 25, 1). Take the blood to be investigated (about 0'5 c.cm.), and dilute it with water until it shows exactly the same optical effects in the spectroscope. If k is the percentage of Hb which allows green to pass](https://iiif.wellcomecollection.org/image/b24757342_0001_0062.jp2/full/800%2C/0/default.jpg)