Volume 1
A textbook of human physiology / / translated from [the] 7th German edition by William Stirling.
- Landois, Leonard
- Date:
- 1891
Licence: Public Domain Mark
Credit: A textbook of human physiology / / translated from [the] 7th German edition by William Stirling. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
67/602 (page 27)
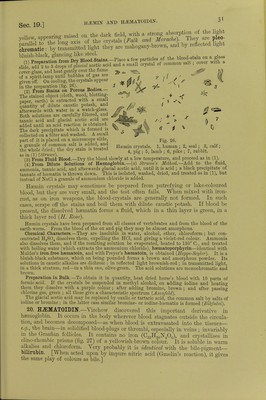
![REDUCED HAEMOGLOBIN. absorption-band, is formed. The colour changes from a bright red to a purplish o! cS tint. The two hands are reproduced by shaking the reduced hemoglobin with air, whereby Hb02 is again formed. Solutions of oxyhemoglobin are readily ! nguished by their scarlet colour from the purplish tint of reduced hemoglobin TThe single absorption-band (fig. 23, 4), designated by the letter (y), lying about midway between the position of the two previous bands, is broader, fainter, less uCB D Figs. 24 ami 25. Fig. 24, graphic representation of the spectrum of Hb02. Fig. 25, the same of Hb, showing the amount of absorption with varying strengths of hemoglobin, the thickness of the fluid remaining the same. The numbers indicate the percentage of colouring matter. deeply shaded, and its centre is about, but not quite, intermediate between D and E. It extends between the wave-lengths 595 and 538, and is blackest opposite the wave-length 550, so that it lies nearer D than E. At the same time more of the blue rays are transmitted. On dilution the. band is not resolved into two, but simply becomes fainter and disappears.] [According to Hermann, the absorption-band of Hb is not a single band, there being in addition a very narrow band towards the red end of the spectrum, but separate from the chief absorption-band by a very small interval.] [Hemoglobin has certain remarkable characters :—(1) Although it is a crystalloid body it diffuses with difficulty through an animal membrane, owing to the large size of its molecule. (2) It readily combines with 0 to form an vmstable and loose chemical compound, oxyhemoglobin. (3) This 0 it gives up readily to the tissues or other deoxidising reagents. (4) Its composition is very complex, for, in addition to the ordinary elements present in proteids, it contains a remarkable amount of iron (0*4 per cent).] If a string be tied round the base of two fingers so as to interrupt the circulation, spectro- scopic examination shows that the oxyhemoglobin rapidly passes into reduced Hb (Vicrordt). Cold delays this reduction ; it is accelerated in youth, during muscular activity, or by suppressed respiration, and usually also during fever. The spectroscopic examination of small blood-stains is often of the utmost forensic import- ance. A minimal drop is sufficient. Dissolve the stain in a few drops of distilled water, and place the solution in a thin glass tube in front of the slit of the spectroscope. Para-haemoglobin.—If Hb02 be preserved under alcohol it passes into a modified form, which is insoluble in water (Ncncki and Sicbcr). 2. Methsemoglobin is a more stable, crystalline compound {Hoppe-Seyler). It contains the same amount of 0 as Hb02, but in a different chemical union, while the 0 is also more firmly united with it. It shows four absorption-bands like hematin in acid solution (fig. 23, 5), of which that between C and D is distinct; the second is very indistinct, while the third and fourth readily fuse, so that these last two bands are only well seen with good apparatus. It is produced spontaneously in old brown blood-stains, in the crusts of bloody wounds, in cysts with sanguinolent contents, and in bloody urine. Chemically, it can be prepared from](https://iiif.wellcomecollection.org/image/b24757342_0001_0067.jp2/full/800%2C/0/default.jpg)