Volume 1
A textbook of human physiology / / translated from [the] 7th German edition by William Stirling.
- Landois, Leonard
- Date:
- 1891
Licence: Public Domain Mark
Credit: A textbook of human physiology / / translated from [the] 7th German edition by William Stirling. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
68/602 (page 28)
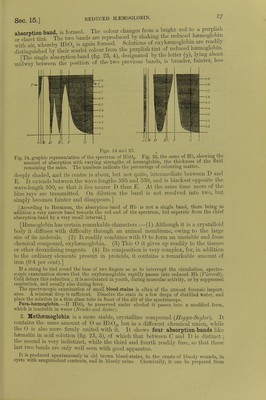
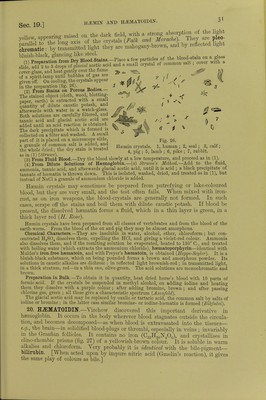
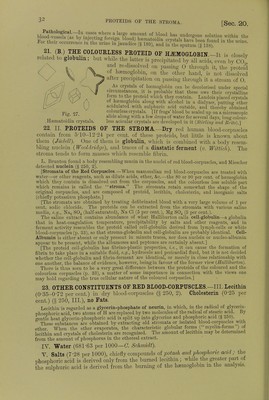
![an I the malmgany-brown laky Hind be a lowed to evaporate slowly (Halliburton) * S^^L:r»«a: red- If-^—^'ide be aaaiSSS [Action of Nitrites.-The addition of amyl nitrite dissolved in alcohol, or sodic or potassic nitrite to defibrinated blood causes the latter to assume a chocolate colour, winch, on the addition of ammonia, changes to red. The chocolate-coloured fluid shows one well-defined band in the red, and less distinctly other three bands like metnsemogloDin (Gamgee).] [The nitrites therefore form a compound with its oxygen more firmly fixed than the 0 in ribU,, so that large closes of nitrites arrest the internal respiration and are poisonous. It is however, affected by the products formed in the blood during asphyxia, while CO-Hb is not the methamioglobin formed by the nitrites is reduced by these products to Hb,. which as it passes through the lungs takes up 0.] 16. CAEBONIC OXIDE-HEMOGLOBIN, POISONING WITH CO.—3. CO- Haemoglobin is a more stable chemical compound than the foregoing, and is pro- duced at once when carbonic oxide is brought into contact with pure Hb or Hb02 (CI Bernard, 1857). It has an intensely florid or cherry-red colour, is not dichroic, and its spectrum shows two absorption-bands, very like those of Hb02, but they are slightly closer together and lie more towards the violet (fig. 23, 3). Reducing substances which act upon Hb02, e.g., ammonium sulphide or Stokes's fluid, do not affect these bands, i.e., they cannot convert the CO-Hb into reduced Hb. If a 10 per cent, solution of caustic soda be added to a solution of CO-Hb, and heated, it gives a cinnabar-red colour; while, with an Hb02 solution, it gives a dark brown, greenish, greasy mass. Spectrum analysis and the soda test enable one to distinguish ^ HbCO, mixed with ^ Hb02. Oxidising substances [solutions of potassic permanganate (0-025 per cent.), potassic chlorate (5 per cent.), and dilute chlorine solution] make solutions of CO-Hb cherry-red in colour, while they turn solutions of Hb02 pale yellow. After this treatment both solutions show the absorption-bands of methaemoglobin, but those of the CO-Hb appear consider- ably later. If ammonium sulphide be added, Hb02 and CO-Hb are re-formed. Hb-CO Reactions.—Modified Soda Test.—Dilute the blood 20 times and add an equal volume of caustic soda (S. G. 1340) (Salkoivski). [Dilute 1 c.cm. HbCO with 50 c.cm. of water, to 10 c.cm. of this mixture add 0*2 c.cm. orange-coloured ammonium sulphide (2 grnis. of sulphur are added to 100 c.cm. yellow ammonium sulphide), and then 02 c.cm. of 30 per cent, acetic acid. The HbCO blood becomes bright red, while normal blood becomes greenish-gray (Katayama).] On account of its stability, CO-Hb resists external influences and even putrefaction for a long time, and the two bands of the spectrum may be visible after many months. Landois obtained the soda test and spectroscopic bands in the blood of a woman poisoned eighteen months pre- viously by CO, and after great rmtrefaction of the body had taken place. [Stirling has kept CO-HI) in a stoppered bottle for five years without putrefaction taking place.] If CO or air containing it be inspired, it gradually displaces the O, volume for volume, out of the red blood-corpuscles, and death soon occurs; 1000 c.cm. inspired at once will kill a man. A very small quantity in the air - 10100) suffices, in a relatively short time, to form a large quantity of CO-Hb. As continued contact with other gases (such as the passing of O through it for a very long time) gradually separates the CO from the Hb, with the formation of Hb02, it happens that, in very partial poisoning with CO, the blood gradually gets rid of the CO by the respiratory organs. It does not appear that any part is further oxidised into CO., in the organism. [CO-hsemoglobin, being a stable compound when once formed, circulates in the blood-vessels; but it neither gives up oxygen to the tissues, nor takes up oxygen in the lungs, hence its very poisonous properties.](https://iiif.wellcomecollection.org/image/b24757342_0001_0068.jp2/full/800%2C/0/default.jpg)