Volume 1
Encyclopædia of chemistry, theoretical, practical, and analytical, as applied to the arts and manufacturers / by writers of eminence. Illustrated with numerous wood-cuts and steel-plate engravings.
- Date:
- 1877-1879
Licence: Public Domain Mark
Credit: Encyclopædia of chemistry, theoretical, practical, and analytical, as applied to the arts and manufacturers / by writers of eminence. Illustrated with numerous wood-cuts and steel-plate engravings. Source: Wellcome Collection.
32/1132 page 16
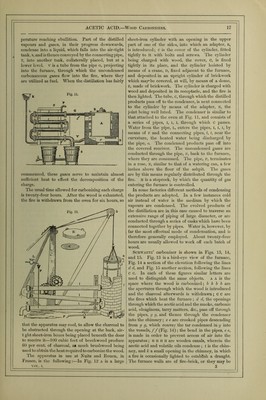
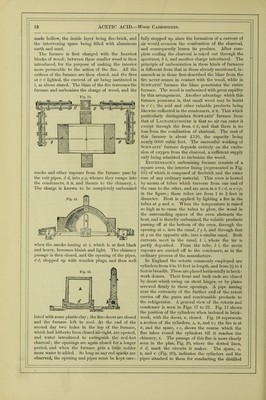
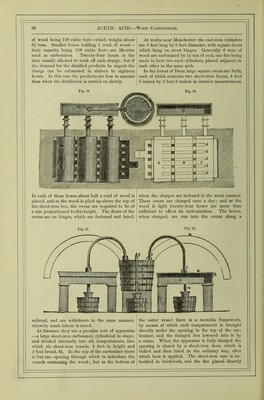
![ment in presence of lime, at the temperature of 96° Fahr. (35°’5 C.), acetic acid is generated in large quantities, and is, after subsidence of the action, found united with the alkaline earth in the form of acetate of lime. In three experiments with different varieties of sea-weed, he obtained as a result an aver- age quantity of 1‘8 per cent, of anhydrous acetic acid. He employed a temperature of 9G“ Fahr. (35°'5 C.), and added hydrate of lime gradually, keeping the mass slightly alkaline, till the fermentation ceased; after which the liquid was filtered off, evaporated to drjmess, and the residue heated (to decompose the mucilaginous matters), when crude acetate of lime remained. This process has never yet been carried out on a manufacturing scale, although in some of the northern countries of Europe, as well as on some of the Scottish and Irish coasts, where sea-weed is plentiful, vinegar might be profitably produced. PYROLIGNEOUS ACID, or Wood Vbmiar.— Actdum Pyrolignosum, Latin; Holz-sciiire, or Holz-essig, German; Acide pyroligneux, or Vinaigre de Bois, French.—The term pyroligneous acid is purely tech- nical, and merely implies the crude acetic acid, con- taminated with various empyreumatic oils, which is obtained from wood by destructive distillation. All organic bodies, except those which sublime without change, are decomposed when exposed to heat in closed vessels, their constituents interchanging with each other and forming new compounds, which are of sufficient stability to resist the particular tempera- ture employed. Thus the elementary components of wood, after a certain amount of heat is applied, arrange themselves into combinations quite distinct from those in which they originally were. Some of these are gaseous; but at moderate temperatures by far the greater part are liquid, the quantity of the latter depending entirely upon the greater or less degree of heat applied in the distillation. The main cause of decomposition of such an organic body as wood by heat is, that the strong affinity of its contained oxygen for carbon and hydrogen, and the comparatively great stability of the more simple compounds of these bodies, causes their formation immediately that there is a sufficient amount of com- motion created amongst the atoms of the original body to allow them to commingle freely. Heat sets up the necessary amount of vibration, and those compounds are at once formed which can resist, vdthout rupture of their constituents from each other, the multitude or amplitude of the vibrations corre- sponding to the temperature at which they are evolved. As a general rule, those bodies containing much oxygen are decomposed at comparatively low tem- ] < rvtures. Acetic acid is an exception ; as has been before observed, a dull red heat does not cause its constituents to fly sufficiently apart from each other to cause their total separation, and the conqiound therefore remains unchanged. To this circumstance is due the large amount of acetic acid which is pro- duced during the destructive distillation of wood. The composition of wood may be taken as CgHj^Oj (or cellulose, also called lignin. The hydrogen and oxygen being in the proportions to form water, the withdrawal of carbon would form acetic acid thus:—SCgHj^Oj — 2C = 4C„H402‘ As might be anticipated, acetic acid is amongst the earliest and most abundant products of the distilla- tion of wood, and being volatile, escapes decomposi- tion at the higher temperatures employed later. As the distillation progresses, marsh gas (CH^), olefiant gas (C2II4), tetrylene (C^Hg), and volatile oils, such as benzol (Cgllg), toluol (C.^Hg), naphthalm (Cj^Hg), paraffin (C.^pH^,), phenol (CgHgO), &c., are given off. At the close of the operation nothing but char- coal remains. The actual facts which are observed in the distillation of wood are as follows:—First, the water passes off, which is extraneous to the wood; secondly, the wood itself is decomposed, and gives rise to water and the crude acetic acid, which is next eliminated; thirdly, condensable matters containing an excess of carbon, forming the tar and oily sub- stance, pass over; and lastly, towards the close of the distOlation, carbonic oxide and marsh gas are evolved, leaving in the retort a charcoal similar in form to the wood introduced. The distillation of wood is earned on in large cast- iron cylinders, or in square ovens made of stout sheet-iron, riveted firndy together, the heat being applied to them directly. A convenient apparatus for the distillation of wood is sketched below. Fig. 10 is the plan, and fig. 11 the elevation of Fig. 10. the apparatus. In these figures, a. is a box made of cast-iron plates, firmly bolted together, of the capacity of 100 cubic feet. This box is imbedded in brickwork. B is a cover on the upper end of the box, through which the charge of wood is introduced, another such opening being at tlie opposite side (not shown in the figures), and is made air-tight by a cover such as B. Through this second door the charcoal is Avithdrawn. c, the firebars; D, the firedoor, through which the fuel is introduced; e e, the spiral course of the flame round the box, and//, the flue passing into the chimney. The iron pipe, G, conducts the gases and other volatile matters evolved to the condenser, which consists of a series of pipes, i, i, i, of large calibre, through which the pilie, G, passes, leaving a sur- rounding space through which cold water con- stantly floAvs. The pipes rest one above the other on a Avooden frameAvork, H. Through L a stream of Avater from the tank, K, enters the loAver condens- ing pipe, and floAvs thence into the others by the connecting pipes, 0 0, till discharged by P, at a tern-](https://iiif.wellcomecollection.org/image/b28127055_0001_0032.jp2/full/800%2C/0/default.jpg)