The estimation of calcium in blood-serum / by John William Trevan and Henrietta Winifred Bainbridge.
- Trevan, J. W. (John William)
- Date:
- [1926?]
Licence: In copyright
Credit: The estimation of calcium in blood-serum / by John William Trevan and Henrietta Winifred Bainbridge. Source: Wellcome Collection.
4/4 (page 426)
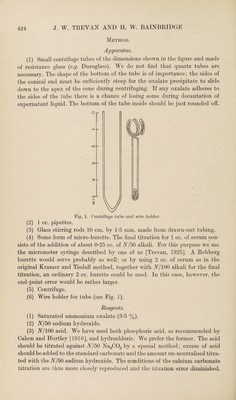
![stirring rods and then transferring it to the solution. The smallest drop which can be dealt with in this way has a volume of about 0-00015 cc., which is more than sufficiently small for accurate titration. This corresponds to about half a division on a metric micrometer head for the average “tuberculin syringe.” The formation of drops of this size and their adequate removal by the stirring rod are much facilitated by coating the exterior of the needle of the syringe with paraffin wax, as recommended by Wright and Colebrook [1921]. The needle may be either the finest steel hypodermic needle or the glass needle described with the micrometer syringe. The latter is preferable as it is easier to see that it is filled to the tip when the titration is begun. Titration is carried to the point where the solution has a colour intermediate between those of buffers of pjj 4-0 and 4-2 each with a concentration of bromophenol blue equal to that of the solution which is being titrated. The difference between the titration figure so obtained and the titration figure for the acid alone gives the amount of calcium in the serum taken. Results. Solution of calcium chloride containing 0-091 mg. per cc. made by dissolving calcium carbonate in hydrochloric acid: 0-09140, 0-0918, 0-0916, 0-0914 mg. Ca per cc. Horse serum: 8-6, 8-54 mg. Ca per 100 cc. on the same sample. Rabbit serum: 13-26, 13-20, 13-16 mg. Ca per 100 cc. We have obtained figures within less than 5 % of the correct value when using the same method with 0-01 mg. Ca per cc., measuring all solutions with a micrometer syringe and keeping the tubes covered during standing and centrifugation with rubber caps, such as are used for vaccine bottles. We have, however, not often used these small quantities. Summary. A method of estimating calcium is described in which the calcium is pre¬ cipitated as oxalate and converted into calcium carbonate by heating the centrifuge tube in which it is separated and washed. The calcium carbonate is titrated with acid. The method has the advantages that the end point is more distinct than that of a permanganate titration, and all washing is carried out with saturated ammonium oxalate, thus avoiding errors due to the solubility of calcium oxalate in water. By the use of the micrometer syringe 1 cc. only of serum is required and smaller quantities down to 0-1 cc. will give results accurate enough for most purposes. KEFERENCES. Cahen and Hurtley (1916). Biochem. J. 10, 308. Clark and Collip (1925). J. Biol. Chem. 63, 461. Hamilton (1925). J. Biol. Chem. 65, 101. Kramer and Tisdall (1921). J. Biol. Chem. 47, 475. Erevan (1925). Biochem. J. 19, 1111. Wright and Colebrook (1921). Technique of the Teat and Capillary Tube (London).](https://iiif.wellcomecollection.org/image/b30625993_0004.jp2/full/800%2C/0/default.jpg)